Efficacy of Using Dendritic Cells in the Treatment of Prostate Cancer: A Systematic Review
- PMID: 40430079
- PMCID: PMC12112211
- DOI: 10.3390/ijms26104939
Efficacy of Using Dendritic Cells in the Treatment of Prostate Cancer: A Systematic Review
Abstract
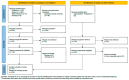
(1) The primary prostate cancer treatment involves androgen deprivation therapy, with or without chemotherapy. Immunotherapy has emerged as a promising strategy against cancer due to its ability to modulate the immune system, overcome immune evasion, and stimulate the attack on tumor cells. Thus, this review urges an exploration of the underlying mechanisms to validate the efficacy and safety of dendritic cell immunotherapy for prostate cancer treatment. (2) An extensive literature search identified 45 eligible studies in PubMed, Web of Science, SCOPUS, and Embase databases. Phase I and II clinical trials and in vitro studies (PROSPERO registration number CRD42024538296) were analyzed to extract information on patient selection, vaccine preparation, treatment details, and disease progression. (3) Despite significant variability in vaccine development and treatment protocols, vaccines were shown to induce satisfactory immune responses, including T-cell activation, increased CD4 and CD8 cell populations, upregulated expression of HLA-A2 and HLA-DR, enhanced migratory capacity of dendritic cells, and elevated interferon levels. Cytokine responses, particularly involving Interleukin 10 (IL-10) and Interleukin 12 (IL-12), varied across studies. Immunotherapy demonstrated potential by eliciting positive immune responses, reducing PSA levels, and showing an acceptable safety profile. However, side effects such as erythema and fever were observed. (4) The analyzed treatments were well-tolerated, but variability in clinical responses and side effects underscores the need for further research to optimize the efficacy and safety of this therapeutic approach.
Keywords: cancer; immune response; immunotherapy; lymphocytes; vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Early versus deferred androgen suppression in the treatment of advanced prostatic cancer.Cochrane Database Syst Rev. 2002;(1):CD003506. doi: 10.1002/14651858.CD003506. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2019 Jun 11;6:CD003506. doi: 10.1002/14651858.CD003506.pub2. PMID: 11869665 Updated.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
References
-
- Bergengren O., Pekala K.R., Matsoukas K., Fainberg J., Mungovan S.F., Bratt O., Bray F., Brawley O., Luckenbaugh A.N., Mucci L., et al. 2022 Update on Prostate Cancer Epidemiology and Risk Factors—A Systematic Review. Eur. Urol. 2023;84:191–206. doi: 10.1016/j.eururo.2023.04.021. - DOI - PMC - PubMed
-
- World Health Organization Global Health Estimates. World Health Organization; Geneva, Switzerland: 2022.
-
- Horn L., Spigel D.R., Vokes E.E., Holgado E., Ready N., Steins M., Poddubskaya E., Borghaei H., Felip E., Paz-Ares L., et al. Journal of clinical oncology Nivolumab Versus Docetaxel in Previously Treated Patients with Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057) J. Clin. Oncol. 2017;35:3924–3933. doi: 10.1200/JCO.2017.74.3062. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

