Characterization of Novel and Known Activators of Cannabinoid Receptor Subtype 2 Reveals Mixed Pharmacology That Differentiates Mycophenolate Mofetil and GW-842,166X from MDA7
- PMID: 40430094
- PMCID: PMC12112332
- DOI: 10.3390/ijms26104956
Characterization of Novel and Known Activators of Cannabinoid Receptor Subtype 2 Reveals Mixed Pharmacology That Differentiates Mycophenolate Mofetil and GW-842,166X from MDA7
Abstract
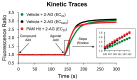
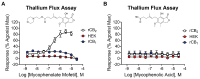
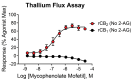
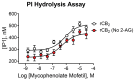
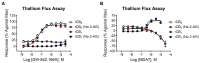
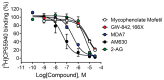
CB1 and CB2 cannabinoid receptors are members of the GPCR superfamily that modulate the effects of endocannabinoids. CB1 is the most abundant CB receptor in the central nervous system, while CB2 is present both peripherally and in the brain. CB2 plays a role in inflammation, as well as neurodegenerative and psychiatric disorders. To identify new ligands for CB2, we screened a library of FDA-approved drugs for activity at the receptor using a thallium flux assay, resulting in the discovery of the immunosuppressant mycophenolate mofetil as a potent, selective activator of CB2. Further characterization of the compound confirmed agonist activity in a variety of complementary assays, including PI hydrolysis, cAMP inhibition, and β-arrestin recruitment. Radioligand binding assays established a non-competitive interaction with the site occupied by [3H]CP55,940. CB2 agonists GW-842,166X and MDA7 were also profiled, revealing that GW-842,166X exhibits a similar activity profile to mycophenolate mofetil, whereas MDA7 presents a distinct profile. These differences provide insight into the complex CB2 pharmacology impacting preclinical and clinical studies, and ultimately, new treatment strategies for brain disorders.
Keywords: GW-842,166X; MDA7; allosteric modulator; brain disorders; cannabinoid receptor; mycophenolate mofetil.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors.Br J Pharmacol. 2019 May;176(10):1455-1469. doi: 10.1111/bph.14440. Epub 2018 Aug 10. Br J Pharmacol. 2019. PMID: 29981240 Free PMC article.
-
Negative allosteric modulators of cannabinoid receptor 2: protein modeling, binding site identification and molecular dynamics simulations in the presence of an orthosteric agonist.J Biomol Struct Dyn. 2020 Jan;38(1):32-47. doi: 10.1080/07391102.2019.1567384. Epub 2019 Feb 5. J Biomol Struct Dyn. 2020. PMID: 30652534 Free PMC article.
-
Pharmacological data of cannabidiol- and cannabigerol-type phytocannabinoids acting on cannabinoid CB1, CB2 and CB1/CB2 heteromer receptors.Pharmacol Res. 2020 Sep;159:104940. doi: 10.1016/j.phrs.2020.104940. Epub 2020 May 26. Pharmacol Res. 2020. PMID: 32470563
-
Latest progress in the identification of novel synthetic ligands for the cannabinoid CB2 receptor.Mini Rev Med Chem. 2014 May;14(5):426-43. doi: 10.2174/1389557514666140428105753. Mini Rev Med Chem. 2014. PMID: 24766386 Review.
-
Cannabinoid CB1 and CB2 receptor ligand specificity and the development of CB2-selective agonists.Curr Med Chem. 2008;15(14):1428-43. doi: 10.2174/092986708784567716. Curr Med Chem. 2008. PMID: 18537620 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

