Isolation, Sphalerite Bioleaching, and Whole Genome Sequencing of Acidithiobacillus ferriphilus QBS3 from Zinc-Rich Sulfide Mine Drainage
- PMID: 40430218
- PMCID: PMC12113256
- DOI: 10.3390/life15050792
Isolation, Sphalerite Bioleaching, and Whole Genome Sequencing of Acidithiobacillus ferriphilus QBS3 from Zinc-Rich Sulfide Mine Drainage
Abstract
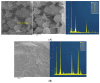
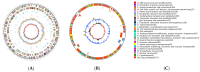
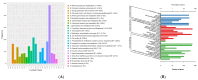
The genus Acidithiobacillus has been widely used in bioleaching, and novel strains in this genus, such as A. ferriphilus, have also been confirmed to possess bioleaching capabilities. In this study, an Acidithiobacillus ferriphilus strain, QBS3, was isolated from zinc-rich sulfide mine drainage using the gradient dilution method. QBS3 is a Gram-negative, 1.3 µm rod-shaped bacterium with small red colonies. It showed a high iron oxidation efficiency of 0.361 g/(L·h) and a sulfur oxidation efficiency of 0.206 g/(L·d). QBS3 has sphalerite bioleaching ability; using QBS3 for pure sphalerite bioleaching, 18.8% of zinc was extracted in 14 days at 1% pulp density. Whole genome sequencing was performed on QBS3. Functional prediction showed that 9.13% of the genes were involved in replication, recombination, and repair. Bioleaching-related genes were analyzed, including iron and sulfur oxidation genes, and carbon and nitrogen fixation genes. For iron oxidation, the Cyc2→RusA pathway and Iro→RusB pathway were found in QBS3. In terms of sulfur oxidation, QBS3 has an incomplete SOX system and lacks the SDO gene, but Rho and Trx may complement the SOX system, enabling QBS3 to oxidize sulfur. QBS3 has multiple sets of carbon fixation genes, and nitrogen fixation genes were also identified. A hypothetical sphalerite bioleaching model is proposed; this study provides a theoretical basis for the zinc sulfide ore bioleaching industry.
Keywords: Acidithiobacillus ferriphilus; bioleaching; ferrous oxidation; isolation; sphalerite; sulfur oxidation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









Similar articles
-
Comparison of bioleaching behaviors of different compositional sphalerite using Leptospirillum ferriphilum, Acidithiobacillus ferrooxidans and Acidithiobacillus caldus.J Ind Microbiol Biotechnol. 2009 Jun;36(6):845-51. doi: 10.1007/s10295-009-0560-9. Epub 2009 Mar 31. J Ind Microbiol Biotechnol. 2009. PMID: 19333635
-
Biooxidation of Arsenopyrite by Acidithiobacillus ferriphilus QBS 3 Exhibits Arsenic Resistance Under Extremely Acidic Bioleaching Conditions.Biology (Basel). 2025 May 15;14(5):550. doi: 10.3390/biology14050550. Biology (Basel). 2025. PMID: 40427739 Free PMC article.
-
Genome sequencing and metabolic network reconstruction of a novel sulfur-oxidizing bacterium Acidithiobacillus Ameehan.Front Microbiol. 2023 Nov 20;14:1277847. doi: 10.3389/fmicb.2023.1277847. eCollection 2023. Front Microbiol. 2023. PMID: 38053556 Free PMC article.
-
From Genes to Bioleaching: Unraveling Sulfur Metabolism in Acidithiobacillus Genus.Genes (Basel). 2023 Sep 8;14(9):1772. doi: 10.3390/genes14091772. Genes (Basel). 2023. PMID: 37761912 Free PMC article. Review.
-
Mechanisms of bioleaching: iron and sulfur oxidation by acidophilic microorganisms.Essays Biochem. 2023 Aug 11;67(4):685-699. doi: 10.1042/EBC20220257. Essays Biochem. 2023. PMID: 37449416 Free PMC article. Review.
References
-
- Shi S., Fang Z., Ni J. Comparative study on the bioleaching of zinc sulphides. Process Biochem. 2006;41:438–446. doi: 10.1016/j.procbio.2005.07.008. - DOI
-
- Ubaldini S., Veglió F., Toro L., Abbruzzese C. Biooxidation of arsenopyrite to improve gold cyanidation: Study of some parameters and comparison with grinding. Int. J. Miner. Process. 1997;52:65–80. doi: 10.1016/S0301-7516(97)00041-0. - DOI
-
- Zou G., Papirio S., Lai X., Wu Z., Zou L., Puhakka J., Ruan R. Column leaching of low-grade sulfide ore from Zijinshan copper mine. Int. J. Miner. Process. 2015;139:11–16. doi: 10.1016/j.minpro.2015.04.005. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

