D1-A-D2 Conjugated Porous Polymers Provide Additional Electron Transfer Pathways for Efficient Photocatalytic Hydrogen Production
- PMID: 40430361
- PMCID: PMC12114361
- DOI: 10.3390/molecules30102190
D1-A-D2 Conjugated Porous Polymers Provide Additional Electron Transfer Pathways for Efficient Photocatalytic Hydrogen Production
Abstract
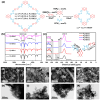
The strategic design of donor-acceptor (D-A) conjugated porous polymers has emerged as a pivotal methodology for advancing efficient photocatalytic hydrogen evolution. However, conventional D-A polymeric architectures face inherent limitations: excessively strong acceptor units may lower the LUMO energy level, compromising proton (H+) reduction capability, while weak D-A interactions result in inadequate light-harvesting capacity and insufficient photogenerated electrons, ultimately diminishing photocatalytic activity. To address these challenges, we developed a new D1-A-D2 conjugated porous polymer (CPP) system. The strategic incorporation of a secondary donor benzothiophene (DBBTh) unit enabled precise bandgap engineering in D1-A-D2 CPPs. Experimental results demonstrate that DBBTh integration significantly enhances both light absorption efficiency and proton reduction ability. Under visible-light irradiation (λ > 420 nm), the Py-BKh1 photocatalyst achieved a hydrogen evolution rate (HER) of 10.2 mmol h-1 g-1 with an apparent quantum yield (AQY) of 9.5% at 500 nm. This work provides a groundbreaking paradigm for designing high-performance organic photocatalysts.
Keywords: D1-A-D2 conjugated polymer; direct C–H arylation polymerization; photocatalysis; visible-light-driven hydrogen evolution; π-conjugated porous polymers.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
-
- Wang W., Tadé M.O., Shao Z. Nitrogen-doped simple and complex oxides for photocatalysis: A review. Prog. Mater. Sci. 2018;92:33–63. doi: 10.1016/j.pmatsci.2017.09.002. - DOI
-
- Cheng L., Xiang Q., Liao Y., Zhang H. CdS-based photocatalysts. Energy Environ. Sci. 2018;11:1362–1391. doi: 10.1039/C7EE03640J. - DOI
-
- Li X., Wu X., Liu S., Li Y., Fan J., Lv K. Effects of fluorine on photocatalysis. Chin. J. Catal. 2020;41:1451–1467. doi: 10.1016/S1872-2067(20)63594-X. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

