A Comprehensive Review of Catalytic Hydrodeoxygenation of Lignin-Derived Phenolics to Aromatics
- PMID: 40430397
- PMCID: PMC12114535
- DOI: 10.3390/molecules30102225
A Comprehensive Review of Catalytic Hydrodeoxygenation of Lignin-Derived Phenolics to Aromatics
Abstract
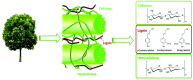
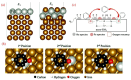
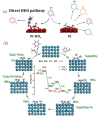
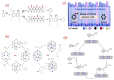
Single-ring aromatic compounds including BTX (benzene, toluene, xylene) serve as essential building blocks for high-performance fuels and specialty chemicals, with extensive applications spanning polymer synthesis, pharmaceutical manufacturing, and aviation fuel formulation. Current industrial production predominantly relies on non-renewable petrochemical feedstocks, posing the dual challenges of resource depletion and environmental sustainability. The catalytic hydrodeoxygenation (HDO) of lignin-derived phenolic substrates emerges as a technologically viable pathway for sustainable aromatic hydrocarbon synthesis, offering critical opportunities for lignin valorization and biorefinery advancement. This article reviews the relevant research on the conversion of lignin-derived phenolic compounds' HDO to benzene and aromatic hydrocarbons, systematically categorizing and summarizing the different types of catalysts and their reaction mechanisms. Furthermore, we propose a strategic framework addressing current technical bottlenecks, highlighting the necessity for the synergistic development of robust heterogeneous catalysts with tailored active sites and energy-efficient process engineering to achieve scalable biomass conversion systems.
Keywords: aromatic hydrocarbon; benzene; electrocatalytic hydrogenation; hydrodeoxygenation; lignin; phenolic compounds.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Ye K., Liu Y., Wu S., Zhuang J. A review for lignin valorization: Challenges and perspectives in catalytic hydrogenolysis. Ind. Crops Prod. 2021;172:114008. doi: 10.1016/j.indcrop.2021.114008. - DOI
-
- Shu R., Li R., Lin B., Wang C., Cheng Z., Chen Y. A review on the catalytic hydrodeoxygenation of lignin-derived phenolic compounds and the conversion of raw lignin to hydrocarbon liquid fuels. Biomass Bioenergy. 2020;132:105432. doi: 10.1016/j.biombioe.2019.105432. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

