De Novo Terpenes Emitted from Juvenile Leaves of Eucalyptus globulus Labill. subsp. globulus
- PMID: 40430405
- PMCID: PMC12114481
- DOI: 10.3390/molecules30102234
De Novo Terpenes Emitted from Juvenile Leaves of Eucalyptus globulus Labill. subsp. globulus
Abstract
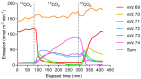
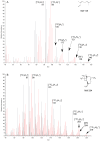
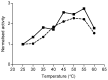
The contributions of de novo synthesis to terpene emissions from Eucalyptus globulus subsp. globulus were determined by fumigating branchlets with 13CO2 in a gas exchange system. Of more than thirty-four terpenes emitted by this species, only four, i.e., isoprene, iso-valeraldehyde, cis-ocimene, and trans-caryophyllene, incorporated 13C into the terpene carbon skeleton during the ~5-6 h experiment. 13C incorporation into isoprene and iso-valeraldehyde reached a maximum of ca. 82% of the carbon skeleton, similar to cis-ocimene, with a maximum of 77% 13C incorporation after ~2.5 h exposure to 13CO2. Only ca. 20% of carbon was labelled in trans-caryophyllene after 5-6 h. the incorporation of 13C was observed only in compounds emitted from leaves, and was not detected in either individual oil glands or in bulk leaf tissue. The results suggest the de novo synthesis of some terpenes (isoprene, cis-ocimene, trans-caryophyllene, and iso-valeraldehyde) and their emission is independent of emissions of terpenes stored in oil glands.
Keywords: 13C incorporation; BVOC; Eucalyptus globulus; GC-MS; PTR-MS; de novo synthesis; terpene emissions.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Guenther A., Hewitt C.N., Erickson D., Fall R., Geron C., Graedel T., Harley P., Klinger L., Lerdau M., McKay W.A., et al. A global model of natural volatile organic compound emissions. J. Geophys. Res. Atmos. 1995;100:8873–8892. doi: 10.1029/94JD02950. - DOI
-
- Atkinson R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000;34:2063–2101. doi: 10.1016/S1352-2310(99)00460-4. - DOI
-
- Guenther A., Karl T., Harley P., Wiedinmyer C., Palmer P.I., Geron C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature) Atmos. Chem. Phys. 2006;6:3181–3210. doi: 10.5194/acp-6-3181-2006. Corrected in Atmos. Chem. Phys. 2006, 6, 3181–3210. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

