Epigallocatechin Gallate in Camellia sinensis Ameliorates Skin Aging by Reducing Mitochondrial ROS Production
- PMID: 40430436
- PMCID: PMC12114381
- DOI: 10.3390/ph18050612
Epigallocatechin Gallate in Camellia sinensis Ameliorates Skin Aging by Reducing Mitochondrial ROS Production
Abstract
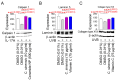
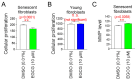
Background: Reactive oxygen species (ROS) generated by mitochondrial dysfunction damage cellular organelles and contribute to skin aging. Therefore, strategies to reduce mitochondrial ROS production are considered important for alleviating skin aging, but no effective methods have been identified. Methods: In this study, we evaluated substances utilized as cosmetic ingredients and discovered Camellia sinensis (C. sinensis) as a substance that reduces mitochondrial ROS levels. Results:C. sinensis extracts were found to act as senolytics that selectively kill senescent fibroblasts containing dysfunctional mitochondria. In addition, C. sinensis extracts facilitated efficient electron transport in the mitochondrial electron transport chain (ETC) by increasing the efficiency of oxidative phosphorylation (OXPHOS), thereby reducing mitochondrial ROS production, a byproduct of the inefficient ETC. This novel mechanism of C. sinensis extracts led to the restoration of skin aging and the skin barrier. Furthermore, epigallocatechin gallate (EGCG) was identified as an active ingredient that plays a key role in C. sinensis extract-mediated skin aging recovery. Indeed, similar to C. sinensis extracts, EGCG reduced ROS and improved skin aging in an artificial skin model. Conclusions: Our data uncovered a novel mechanism by which C. sinensis extract reverses skin aging by reducing mitochondrial ROS production via selective senescent cell death/increased OXPHOS efficiency. Our results suggest that C. sinensis extract or EGCG may be used as a therapeutic agent to reverse skin aging in clinical and cosmetic applications.
Keywords: Camellia sinensis; reactive oxygen species (ROS); senescence rejuvenation; skin aging recovery.
Conflict of interest statement
Authors Eun Young Jeong, Ye Hyang Kim, So Yoon Cha, Ha Yeon Kim, Yeon Kyung Nam, Jin Seong Park, So Yeon Kim and Song Seok Shin was employed by the company Hyundai Bioland Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the paper.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources

