Methionine Dependency and Restriction in Cancer: Exploring the Pathogenic Function and Therapeutic Potential
- PMID: 40430461
- PMCID: PMC12114517
- DOI: 10.3390/ph18050640
Methionine Dependency and Restriction in Cancer: Exploring the Pathogenic Function and Therapeutic Potential
Abstract
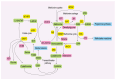
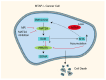
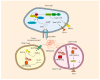
Methionine, an essential amino acid, is obtained by dietary intake to fulfill the requirements of our bodies. Accumulating evidence indicates that methionine plays a pivotal role in various biological processes, including protein synthesis, energy metabolism, redox balance maintenance, and methylation modifications. Numerous advances underscore the heightened dependence of cancer cells on methionine, which is a significant factor in cancer pathogenesis and development. A profound comprehension of the intricate relationship between methionine metabolism and tumorigenesis is imperative for advancing the field of cancer therapeutics. Herein, we delve into the role of methionine in supporting cancer growth, the impact on epigenetic modifications, and the interaction between methionine and the tumor microenvironment. Additionally, we provide insights into the development of various methionine-targeted therapy strategies. This paper summarizes the current state of research and its translational potential, emphasizing the challenges and opportunities associated with harnessing methionine dependence as a target for innovative cancer treatments.
Keywords: cancer; epigenetic modification; methionine metabolism; methionine restriction; tumor microenvironment.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Sugimura T., Birnbaum S.M., Winitz M., Greenstein J.P. Quantitative nutritional studies with water-soluble, chemically defined diets. VIII. The forced feeding of diets each lacking in one essential amino acid. Arch. Biochem. Biophys. 1959;81:448–455. doi: 10.1016/0003-9861(59)90225-5. - DOI - PubMed
-
- Aoki Y., Han Q., Tome Y., Yamamoto J., Kubota Y., Masaki N., Obara K., Hamada K., Wang J.D., Inubushi S., et al. Reversion of methionine addiction of osteosarcoma cells to methionine independence results in loss of malignancy, modulation of the epithelial-mesenchymal phenotype and alteration of histone-H3 lysine-methylation. Front. Oncol. 2022;12:1009548. doi: 10.3389/fonc.2022.1009548. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

