New Challenging Systemic Therapies for Juvenile Scleroderma: A Comprehensive Review
- PMID: 40430462
- PMCID: PMC12114888
- DOI: 10.3390/ph18050643
New Challenging Systemic Therapies for Juvenile Scleroderma: A Comprehensive Review
Abstract
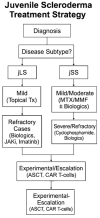
Background: Juvenile scleroderma (JS) comprises a group of rare chronic autoimmune and fibrosing disorders in children, primarily presenting as juvenile localized scleroderma (jLS) or juvenile systemic sclerosis (jSS). While jLS predominantly affects the skin and subcutaneous tissues, jSS may involve multiple internal organs and is associated with increased morbidity and mortality. Due to the scarcity of pediatric-specific clinical trials, the current treatment strategies are largely empirical and often adapted from adult protocols. Objective: This narrative review aims to provide a comprehensive update on emerging systemic therapies for juvenile scleroderma, focusing on biologics, small molecule inhibitors, and advanced cellular interventions, to support the development of more personalized and effective pediatric treatment approaches. Methods: A literature search was conducted through PubMed and a manual bibliographic review, covering publications from 2001 to 2024. Only English-language studies involving pediatric populations were included, comprising randomized controlled trials, reviews, and case reports. Additional searches were performed for drugs that are specifically used in juvenile scleroderma. Results: Biologic agents such as tocilizumab, rituximab, and abatacept, along with small molecules including Janus kinase (JAK) inhibitors and imatinib, have demonstrated potential in managing refractory cases by reducing skin fibrosis and pulmonary involvement. Novel approaches-such as pamrevlumab, nintedanib, and chimeric antigen receptor (CAR-T) cell therapy-target fibrotic and autoimmune pathways but remain investigational in children. Autologous stem cell transplantation (ASCT) has also been explored in severe, treatment-resistant cases, although data are extremely limited. The overall evidence base is constrained by small sample sizes, a lack of controlled pediatric trials, and reliance on adult extrapolation. Conclusions: While innovative systemic therapies show promise for juvenile scleroderma, their widespread clinical application remains limited by insufficient pediatric-specific evidence. Large, multicenter, long-term trials are urgently needed to establish safety, efficacy, and optimal treatment algorithms that are tailored to the pediatric population.
Keywords: childhood; innovative biotechnologies; juvenile scleroderma; personalized medicine; systemic sclerosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Use of biologic drug in the treatment of localized scleroderma and systemic sclerosis in children: A scoping review.Semin Arthritis Rheum. 2025 Apr;71:152634. doi: 10.1016/j.semarthrit.2025.152634. Epub 2025 Jan 29. Semin Arthritis Rheum. 2025. PMID: 39938346
-
Abatacept in the treatment of localized scleroderma: A pediatric case series and systematic literature review.Semin Arthritis Rheum. 2020 Aug;50(4):645-656. doi: 10.1016/j.semarthrit.2020.03.020. Epub 2020 May 19. Semin Arthritis Rheum. 2020. PMID: 32504991 Review.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Juvenile Scleroderma: A Referral Center Experience.Arch Rheumatol. 2018 Jan 18;33(3):344-351. doi: 10.5606/ArchRheumatol.2018.6578. eCollection 2018 Sep. Arch Rheumatol. 2018. PMID: 30632525 Free PMC article.
-
Update on the Systemic Treatment of Pediatric Localized Scleroderma.Paediatr Drugs. 2019 Dec;21(6):461-467. doi: 10.1007/s40272-019-00363-5. Paediatr Drugs. 2019. PMID: 31667717 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

