The Impact of Hepatitis B and/or C on Liver Function and on the Response to Antiretroviral Therapy in HIV-Infected Patients: A Romanian Cohort Study
- PMID: 40430507
- PMCID: PMC12114873
- DOI: 10.3390/ph18050688
The Impact of Hepatitis B and/or C on Liver Function and on the Response to Antiretroviral Therapy in HIV-Infected Patients: A Romanian Cohort Study
Abstract
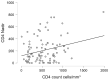
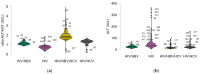
Background: Hepatitis B (HBV) and C (HCV) virus coinfections remain major contributors to liver-related morbidity and mortality among people living with HIV (PLWH). This study aimed to assess the prevalence of HBV and/or HCV coinfections in a Romanian HIV cohort and to evaluate their impact on immunological, virological, and liver function parameters under antiretroviral therapy (ART). Methods: We retrospectively analyzed 462 HIV-infected patients (2018-2021) from the National Institute of Infectious Diseases, Bucharest, stratified into four groups: HIV mono-infection (n = 176), HIV/HBV (n = 114), HIV/HCV (n = 97), and HIV/HBV/HCV (n = 75) coinfections. Immunological (CD4 count, CD8 count, and CD4/CD8 ratio), virological (HIV-1 RNA), and hepatic parameters (ALT, AST, GGT, bilirubin, amylase, and lipase) were compared. Results: No significant differences were observed between groups regarding the immune recovery (mean CD4 count p = 0.89, HIV-RNA suppression p = 0.78). However, liver and pancreatic parameters showed statistically significant deterioration in the coinfected groups. ALT (p < 0.001), GGT (p = 0.009), total bilirubin (p = 0.011), amylase (p = 0.010), and lipase (p < 0.001) were significantly higher in the triple-infection (HIV/HBV/HCV) group compared to HIV mono-infected patients. Coinfection was also associated with a longer duration of illness (p = 0.002) and therapy (p = 0.021) and with a higher number of ART regimens used (p = 0.013). Conclusions: While HIV suppression and immune recovery were not significantly impaired by HBV/HCV coinfections, liver and pancreatic injuries were significantly more prevalent and severe in coinfected patients. Regular monitoring of hepatic function and integrated management strategies are recommended to minimize liver-related complications in this population.
Keywords: HIV; antiretroviral therapy; coinfections; hepatitis B; hepatitis C; immune recovery; liver and pancreatic impairment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
The impact of HBV or HCV infection in a cohort of HIV-infected pregnant women receiving a nevirapine-based antiretroviral regimen in Malawi.BMC Infect Dis. 2014 Apr 4;14:180. doi: 10.1186/1471-2334-14-180. BMC Infect Dis. 2014. PMID: 24708626 Free PMC article.
-
Hepatotoxicity and effectiveness of a Nevirapine-based antiretroviral therapy in HIV-infected patients with or without viral hepatitis B or C infection in Cameroon.BMC Public Health. 2010 Mar 1;10:105. doi: 10.1186/1471-2458-10-105. BMC Public Health. 2010. PMID: 20193053 Free PMC article.
-
Seroprevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) among human immunodeficiency virus (HIV)-infected patients in an HBV endemic area in Brazil.PLoS One. 2018 Sep 7;13(9):e0203272. doi: 10.1371/journal.pone.0203272. eCollection 2018. PLoS One. 2018. PMID: 30192795 Free PMC article.
-
Coinfection of Schistosoma Species with Hepatitis B or Hepatitis C Viruses.Adv Parasitol. 2016;91:111-231. doi: 10.1016/bs.apar.2015.12.003. Epub 2016 Feb 5. Adv Parasitol. 2016. PMID: 27015949 Review.
-
Impact and management of hepatitis B and hepatitis C virus co-infection in HIV patients.Trop Gastroenterol. 2008 Jul-Sep;29(3):136-47. Trop Gastroenterol. 2008. PMID: 19115605 Review.
Cited by
-
From Management to Cure: The Shifting Paradigm in HIV and Chronic Viral Hepatitis.Pharmaceuticals (Basel). 2025 Jul 11;18(7):1034. doi: 10.3390/ph18071034. Pharmaceuticals (Basel). 2025. PMID: 40732322 Free PMC article.
References
-
- Hagos Y.M., Yalew G.T., Meles H.N., Tsegay E., Lemelem M., Wasihun A.G. Hepatitis B and C Viral Coinfection and Associated Factors among HIV-Positive Patients Attending ART Clinics of Afar Regional State, Northeast Ethiopia. PLoS ONE. 2024;19:e0302453. doi: 10.1371/journal.pone.0302453. - DOI - PMC - PubMed
-
- Global Hepatitis Report 2024: Action for Access in Low- and Middle-Income Countries. [(accessed on 29 March 2025)]. Available online: https://www.who.int/publications/i/item/9789240091672.
-
- Global Burden of Hepatitis B Infection in People Living with Human Immunodeficiency Virus: A Systematic Review and Meta-Analysis—PubMed. [(accessed on 29 March 2025)]; Available online: https://pubmed.ncbi.nlm.nih.gov/31813969/ - PubMed
-
- Spradling P.R., Richardson J.T., Buchacz K., Moorman A.C., Brooks J.T. HIV Outpatient Study (HOPS) Investigators Prevalence of Chronic Hepatitis B Virus Infection among Patients in the HIV Outpatient Study, 1996–2007. J. Viral Hepat. 2010;17:879–886. doi: 10.1111/j.1365-2893.2009.01249.x. - DOI - PubMed
-
- Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents With HIV. [(accessed on 29 March 2025)]; Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult....
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

