Design, Synthesis, and Evaluation of Antinociceptive Properties of Novel CBD-Based Terpene-Cinnamoyl-Acyl-Hydrazone Analogues
- PMID: 40430572
- PMCID: PMC12114903
- DOI: 10.3390/ph18050755
Design, Synthesis, and Evaluation of Antinociceptive Properties of Novel CBD-Based Terpene-Cinnamoyl-Acyl-Hydrazone Analogues
Abstract
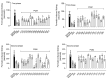
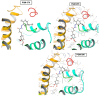
Background/Objectives: Cannabidiol (CBD) has been reported for its antinociceptive, anti-inflammatory, and neuroprotective activities. However, several legal restrictions on its medicinal uses and even research have contributed to the development of synthetic analogues. Therefore, the aim of this study was the design and synthesis of a novel series of CBD-based structural analogues, and the in vivo evaluation of their potential antinociceptive activity. Methods: Using a two-step synthetic route, 26 new terpene-cinnamoyl acyl-hydrazone analogues were obtained and were submitted to in vivo screening in the classical formalin-induced paw edema and hot plate assays. Results: The compounds PQM-292, PQM-293, PQM-295, PQM-307, PQM-308, and PQM-309 exhibited the best results in the neurogenic phase (first phase) of the formalin-induced licking response, showing comparable results to morphine. Notably, in the inflammatory phase (second phase), compound PQM-292 exhibited the best anti-inflammatory activity. Interestingly, in the hot plate model, six other compounds (PQM-274, PQM-291, PQM-294, PQM-304, PQM-305, and PQM-378) showed the best antinociceptive activity in comparison to morphine, especially PQM-274, which exhibited an antinociceptive effect almost equivalent to the reference drug. Interestingly, these findings suggested that these bioactive compounds, despite their structural similarity, act through different mechanisms, which were investigated by molecular docking with CB1, CB2, and TRPV1 receptors. In silico results indicated that the most active compounds should act through different mechanisms, probably involving interactions with TRPA1. Conclusions: Therefore, due to the promising antinociceptive activity observed for these highlighted compounds, particularly for PQM-292 and PQM-274, without apparent toxicity and psychoactive effects, and the possible involvement of diverse mechanisms of action, these compounds could be considered as promising starting points to the development of new drug candidate prototypes of clinical interest.
Keywords: CBD analogues; acute pain; antinociceptive activity; bioactive acyl-hydrazone derivatives; cannabidiol-based analogues.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
Grants and funding
- CNPq, #406739-2018-8, # 308557/2021-2; #306900/2023-8/National Council for Scientific and Technological Development
- FAPEMIG, #APQ-CEX00518-17/Fundação de Amparo à Pesquisa do Estado de Minas Gerais
- FAPERJ, # SEI-260003/003464/2022; SEI-260003/001154/2023/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- INCT-INOFAR, #465249/2014-0, #402176/2024-3/National Institute of Science and Technology of Drugs and Medicines
LinkOut - more resources
Full Text Sources

