Molecular Detection of Encephalitozoon cuniculi in Migratory Waterfowl of the Genus Anser (Anseriformes: Anatidae) in Poland
- PMID: 40430809
- PMCID: PMC12114933
- DOI: 10.3390/pathogens14050489
Molecular Detection of Encephalitozoon cuniculi in Migratory Waterfowl of the Genus Anser (Anseriformes: Anatidae) in Poland
Abstract
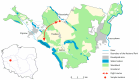
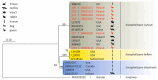
Microsporidiosis is a zoonotic disease that derives from disparate sources. Most of the microsporidial agents are host-specific but some are capable of interspecies transmission, causing disease in various animals including humans. Human microsporidiosis may be caused by 17 species, with Encephalitozoon cuniculi, E. intestinalis and E. hellem mostly being responsible for human infections worldwide. Wildlife and migratory waterfowl can serve as reservoirs of these human-infectious agents and play a significant role in disseminating these pathogens into the environment. The aim of the study was to detect E. cuniculi, E. intestinalis and E. hellem in wild, migratory greater white-fronted geese (Anser albifrons) and other Anatidae members in feacal samples obtained in north-western Poland, using a molecular method. We collected 189 fecal droppings from Anatidae species (75 samples from greater white-fronted geese and 114 from other Anser spp.) during autumn migration. New species specific primers for PCR amplification were used to amplify a fragment of the small subunit ribosomal (SSU) rRNA of E. cuniculi, E. intestinalis and E. hellem. All fecal droppings were negative for E. intestinalis and E. hellem whereas E cuniculi was detected in 6 of 189 fecal samples (3.2%; 95% CI: 1.3-6.3%). In total, 1 of 75 tested fecal samples of greater white-fronted geese was positive (1.3%; 95% CI: 0.08-5.7%) while 5 of 114 (4.4%; 95% CI: 1.6-9.1%) tested fecal samples without exact species affiliation (only Anser sp.) were also positive. The phylogenetic analysis placed the sequences obtained from the birds' droppings in the clade E. cuniculi from various rodents, wild carnivores and humans. Our results provide the first description of the occurrence and genotyping of the microsporidian E. cuniculi in greater white-fronted geese and in other members of the Anserinae Subfamily. Our findings support the results of other authors that E. cuniculi may originate from diverse sources, including common waterfowl. Our results are important in a One Health context, as wild migrating waterfowl may disseminate this zoonotic agent in remote regions through their migratory behaviour. These species should be considered significant sources of zoonotic pathogens, potentially hazardous to domestic and farmed animals as well as humans.
Keywords: encephalitozoonosis; genotyping; microsporidia; molecular epidemiology; zoonosis one health.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

