The Molecular Chaperone TCP1 Affects Carcinogenicity and Is a Potential Therapeutic Target for Acute Myeloid Leukemia
- PMID: 40430849
- PMCID: PMC12114683
- DOI: 10.3390/pharmaceutics17050557
The Molecular Chaperone TCP1 Affects Carcinogenicity and Is a Potential Therapeutic Target for Acute Myeloid Leukemia
Abstract
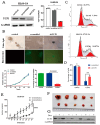
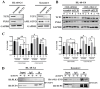
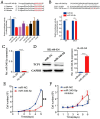
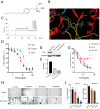
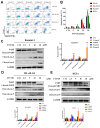
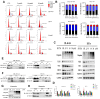
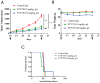
Background/Objectives: Acute myeloid leukemia (AML) is an aggressive malignancy marked by high relapse rates and molecular heterogeneity, necessitating the identification of novel therapeutic targets. T-complex protein 1 (TCP1), a chaperonin implicated in protein folding, remains underexplored in AML pathogenesis. This study investigates the functional role of TCP1 in AML progression and evaluates its therapeutic potential. Methods: Using successive generations of xenografted tumor models, we systematically assessed the correlation between TCP1 expression and AML tumorigenicity. Functional consequences of TCP1 silence were evaluated through in vitro proliferation assays and in vivo tumor growth monitoring. Two distinct inhibitory strategies were employed: miR-340-5p-mediated transcriptional silencing and FTY720-induced disruption of TCP1 chaperone activity. Mechanistic insights were derived from ubiquitin-proteasome pathway analysis, cell cycle profiling, and apoptosis assays. Results: High TCP1 expression correlated strongly with enhanced AML tumorigenicity. Knockdown of TCP1 significantly inhibited AML cell growth and induced degradation of AML1-ETO and PLK1 proteins through the ubiquitin-proteasome pathway. miR-340-5p effectively silenced TCP1 expression, exhibiting an inverse correlation with TCP1 levels. FTY720 disrupted TCP1's chaperone function, leading to cell cycle arrest, apoptosis, and reduced xenograft tumor growth in murine models. Conclusion: Our findings establish TCP1 as a promising therapeutic target for AML. Both miR-340-5p and FTY720 demonstrate potent anti-leukemic effects by suppressing TCP1 activity, highlighting their potential as novel strategies to inhibit AML proliferation and improve therapeutic outcomes.
Keywords: FTY720; TCP1; acute myeloid leukemia; cell cycle arrest; chaperone function; miR-340-5p.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
TCP1 increases drug resistance in acute myeloid leukemia by suppressing autophagy via activating AKT/mTOR signaling.Cell Death Dis. 2021 Nov 8;12(11):1058. doi: 10.1038/s41419-021-04336-w. Cell Death Dis. 2021. PMID: 34750375 Free PMC article.
-
MiR-20a-5p functions as a potent tumor suppressor by targeting PPP6C in acute myeloid leukemia.PLoS One. 2021 Sep 29;16(9):e0256995. doi: 10.1371/journal.pone.0256995. eCollection 2021. PLoS One. 2021. PMID: 34587164 Free PMC article.
-
MiR-1306-5p promotes cell proliferation and inhibits cell apoptosis in acute myeloid leukemia by downregulating PHF6 expression.Leuk Res. 2022 Sep;120:106906. doi: 10.1016/j.leukres.2022.106906. Epub 2022 Jun 23. Leuk Res. 2022. PMID: 35780573
-
Hsa_circ_0044907 promotes acute myeloid leukemia progression through upregulating oncogene KIT via sequestering miR-186-5p.Hematology. 2022 Dec;27(1):960-970. doi: 10.1080/16078454.2022.2113574. Hematology. 2022. PMID: 36004511
-
Human mesenchymal stem cells derived exosomes inhibit the growth of acute myeloid leukemia cells via regulating miR-23b-5p/TRIM14 pathway.Mol Med. 2021 Oct 16;27(1):128. doi: 10.1186/s10020-021-00393-1. Mol Med. 2021. PMID: 34656078 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

