Evaluation of Violacein Metabolic Stability and Metabolite Identification in Human, Mouse, and Rat Liver Microsomes
- PMID: 40430892
- PMCID: PMC12114947
- DOI: 10.3390/pharmaceutics17050601
Evaluation of Violacein Metabolic Stability and Metabolite Identification in Human, Mouse, and Rat Liver Microsomes
Abstract
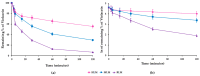
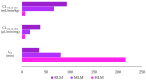
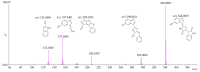
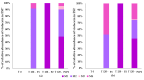
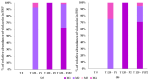
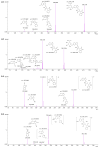
Background: Malaria significantly impacts the health of populations living in poverty and vulnerable conditions. Resistance to current antimalarial drugs remains a major challenge and highlights the urgent need for novel, effective, and safer therapies. Violacein, a purple pigment, has demonstrated potent antiplasmodial activity, making it a promising antimalarial candidate. However, to date, no in vitro metabolism studies of violacein have been published. In this study, the metabolic stability of violacein was evaluated using human (HLMs), mouse (MLMs), and rat (RLMs) liver microsomes and the metabolites generated by HLMs and RLMs were assessed. Methods: Liquid chromatography quadrupole mass spectrometry (LC-MS/MS) was used to investigate the metabolic stability of violacein, while liquid chromatography quadrupole time-of-flight mass spectrometry (LC-QTOF-MS) was used to identify the metabolites. In silico analyses were used to support in vitro metabolite identification by providing insights into potential metabolic pathways and predicting metabolite structures, thereby enhancing the accuracy and efficiency of the identification process. Results: The half-life (t1/2) for violacein in RLMs, MLMs, and HLMs was 36, 81, and 216 min, respectively. The in vitro intrinsic clearance (CLint, in vitro) values were 38.4, 17.0, and 6.4 µL/min/mg for RLMs, MLMs, and HLMs, respectively, while the in vivo intrinsic clearance (CLint, in vivo) was 93.7, 67.0, and 6.6 mL/min/kg, respectively. A slow elimination profile was observed in HLMs followed by MLMs, with rapid elimination in RLMs, indicating greater stability of violacein in HLMs and MLMs when compared with RLMs. Four violacein metabolites were identified in HLMs and RLMs, two of which were formed by phase I metabolism, one by phase II metabolism, and one by phase I + II metabolism. Conclusions: This study provides the first published analysis of the metabolic stability of violacein.
Keywords: ADME; drug development; liver microsomes; malaria; mass spectrometry; metabolic stability; metabolite profiling and stability; violacein.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- World Health Organization . World Malaria Report 2024: Addressing Inequity in the Global Malaria Response. World Health Organization; Geneva, Switzerland: 2024.
-
- Demsash A.W., Worku Z., Shibabaw A.A., Walle A.D., Lemu J.C., Jifar W.W., Bekana T., Gontie G.B., Tesfahun E., Kitil G.W., et al. Pooled prevalence of malaria and associated factors among vulnerable populations in Ethiopia: A systematic review and meta-analysis. BMC Infect. Dis. 2024;24:828. doi: 10.1186/s12879-024-09736-9. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

