Nano-Drug Delivery Systems for Bone Metastases: Targeting the Tumor-Bone Microenvironment
- PMID: 40430894
- PMCID: PMC12115183
- DOI: 10.3390/pharmaceutics17050603
Nano-Drug Delivery Systems for Bone Metastases: Targeting the Tumor-Bone Microenvironment
Abstract
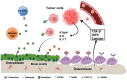
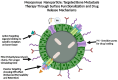
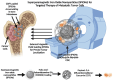
Bone metastases are a prevalent and debilitating consequence of various cancers, including breast and prostate carcinomas, which significantly compromise patient quality of life due to pain, fractures, and other skeletal-related events (SREs). This review examines the pathophysiology of bone metastases, emphasizing the role of the bone microenvironment in tumor progression through mechanisms such as osteotropism and the dysregulated bone remodeling cycle. The primary focus is on the emerging nano-drug delivery systems (DDS) designed to target the bone microenvironment and improve the therapeutic index of anticancer agents. Current treatments, mainly comprising bisphosphonates and radiotherapy, provide palliative benefits but often have limited efficacy and significant side effects. Innovative strategies, such as bisphosphonate-conjugated nanoparticles and targeted therapies that utilize the unique bone marrow niche, are explored for their potential to enhance drug accumulation at metastatic sites while minimizing systemic toxicity. These approaches include the use of liposomes, polymeric nanoparticles, and inorganic nanoparticles, which can be functionalized to exploit the biological barriers within the bone microenvironment. This review also discusses the challenges and future directions for nano-DDS in clinical settings, emphasizing the need for multidisciplinary research to effectively integrate these technologies into standard care protocols.
Keywords: bone metastases; bone-targeting nanoparticles; nano-therapeutics for bone metastasis; nanocarrier systems; nanoparticles in oncology; targeted drug delivery.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

