Enzyme-Based Anti-Inflammatory Therapeutics for Inflammatory Diseases
- PMID: 40430897
- PMCID: PMC12115089
- DOI: 10.3390/pharmaceutics17050606
Enzyme-Based Anti-Inflammatory Therapeutics for Inflammatory Diseases
Abstract
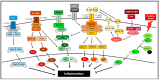
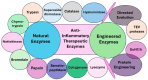
Inflammation is a multifaceted biological response of the immune system against various harmful stimuli, including pathogens (such as bacteria and viruses), cellular damage, toxins, and natural/synthetic irritants. This protective mechanism is essential for eliminating the cause of injury, removing damaged cells, and initiating the repair process. While inflammation is a fundamental component of the body's defense and healing process, its dysregulation can lead to pathological consequences, contributing to various acute and chronic diseases, such as autoimmune disorders, cancer, metabolic syndromes, cardiovascular diseases, neurodegenerative conditions, and other systemic complications. Generally, non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, disease-modifying anti-rheumatic drugs (DMARDs), antihistamines, biologics, and colchicine are used as pharmacological agents in the management of inflammatory diseases. However, these conventional treatments often have limitations, including adverse side effects, long-term toxicity, and drug resistance. In contrast, enzyme-based therapeutics have emerged as a promising alternative due to their high specificity, catalytic efficiency, and ability to modulate inflammatory pathways with reduced side effects. These enzymes function by scavenging reactive oxygen species (ROS), inhibiting cytokine transcription, degrading circulating cytokines, and blocking cytokine release by targeting exocytosis-related receptors. Additionally, their role in tissue repair and regeneration further enhances their therapeutic potential. Most natural anti-inflammatory enzymes belong to the oxidoreductase class, including catalase and superoxide dismutase, as well as hydrolases such as trypsin, chymotrypsin, nattokinase, bromelain, papain, serratiopeptidase, collagenase, hyaluronidase, and lysozyme. Engineered enzymes, such as Tobacco Etch Virus (TEV) protease and botulinum neurotoxin type A (BoNT/A), have also demonstrated significant potential in targeted anti-inflammatory therapies. Recent advancements in enzyme engineering, nanotechnology-based enzyme delivery, and biopharmaceutical formulations have further expanded their applicability in treating inflammatory diseases. This review provides a comprehensive overview of both natural and engineered enzymes, along with their formulations, used as anti-inflammatory therapeutics. It highlights improvements in stability, efficacy, and specificity, as well as minimized immunogenicity, while discussing their mechanisms of action and clinical applications and potential future developments in enzyme-based biomedical therapeutics.
Keywords: engineered enzymes; hydrolases; inflammation; oxidoreductases; pro-inflammatory mediators; protease; therapeutic enzymes.
Conflict of interest statement
No potential conflicts of interest were reported by the author.
Figures

References
-
- Jaeger K.-E., Liese A., Syldatk C. Introduction to Enzyme Technology. Springer; Berlin/Heidelberg, Germany: 2024. Introduction to enzyme technology; pp. 1–16.
Publication types
LinkOut - more resources
Full Text Sources

