Immune Modulation with Oral DNA/RNA Nanoparticles
- PMID: 40430900
- PMCID: PMC12115334
- DOI: 10.3390/pharmaceutics17050609
Immune Modulation with Oral DNA/RNA Nanoparticles
Abstract
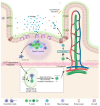
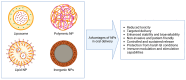
The oral delivery of DNA/RNA nanoparticles represents a transformative approach in immunotherapy and vaccine development. These nanoparticles enable targeted immune modulation by delivering genetic material to specific cells in the gut-associated immune system, triggering both mucosal and systemic immune responses. Unlike parenteral administration, the oral route offers a unique immunological environment that supports both tolerance and activation, depending on the formulation design. This review explores the underlying mechanisms of immune modulation by DNA/RNA nanoparticles, their design and delivery strategies, and recent advances in their application. Emphasis is placed on strategies to overcome physiological barriers such as acidic pH, enzymatic degradation, mucus entrapment, and epithelial tight junctions. Special attention is given to the role of gut-associated lymphoid tissue in mediating immune responses and the therapeutic potential of these systems in oral vaccine platforms, food allergies, autoimmune diseases, and chronic inflammation. Despite challenges, recent advances in nanoparticle formulation support the translation of these technologies into clinical applications for both therapeutic immunomodulation and vaccination.
Keywords: DNA; LNP; RNA; chitosan; drug delivery; immune response; nanoparticles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Advancedoral vaccine delivery strategies for improving the immunity.Adv Drug Deliv Rev. 2021 Oct;177:113928. doi: 10.1016/j.addr.2021.113928. Epub 2021 Aug 16. Adv Drug Deliv Rev. 2021. PMID: 34411689 Review.
-
pH-responsive nanoparticles shelled with chitosan for oral delivery of insulin: from mechanism to therapeutic applications.Acc Chem Res. 2012 Apr 17;45(4):619-29. doi: 10.1021/ar200234q. Epub 2012 Jan 11. Acc Chem Res. 2012. PMID: 22236133 Review.
-
Advances in Oral Subunit Vaccine Design.Vaccines (Basel). 2020 Dec 22;9(1):1. doi: 10.3390/vaccines9010001. Vaccines (Basel). 2020. PMID: 33375151 Free PMC article. Review.
-
Harnessing the potential of the NALT and BALT as targets for immunomodulation using engineering strategies to enhance mucosal uptake.Front Immunol. 2024 Sep 2;15:1419527. doi: 10.3389/fimmu.2024.1419527. eCollection 2024. Front Immunol. 2024. PMID: 39286244 Free PMC article. Review.
-
Lipid Nanoparticle Technology for Clinical Translation of siRNA Therapeutics.Acc Chem Res. 2019 Sep 17;52(9):2435-2444. doi: 10.1021/acs.accounts.9b00368. Epub 2019 Aug 9. Acc Chem Res. 2019. PMID: 31397996 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

