3D Printing of PVA Capsular Devices for Applications in Compounding Pharmacy: Stability Evaluation and In Vivo Performance
- PMID: 40430904
- PMCID: PMC12114815
- DOI: 10.3390/pharmaceutics17050613
3D Printing of PVA Capsular Devices for Applications in Compounding Pharmacy: Stability Evaluation and In Vivo Performance
Abstract
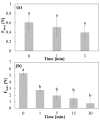
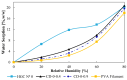
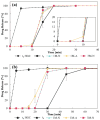
Background: The personalization of medication through 3D printing enables the development of capsular devices (CDs) tailored to patient-specific needs. This study aimed to evaluate the stability and in vivo performance of 3D-printed polyvinyl alcohol (PVA) CDs with 0.4 and 0.9 mm width wall thicknesses (WT) compared to traditional hard gelatin capsules (HGCs). Methods: Capsules were tested for swelling, erosion, adhesion, water sorption, and in vitro disintegration. Additionally, the release of the model drug (losartan potassium) from CDs was evaluated. In vivo capsule opening times were assessed in dogs using X-ray imaging. Stability studies were conducted under natural (25 ± 2 °C, 60 ± 5% RH) and accelerated (40 ± 2 °C, 75 ± 5% RH) storage conditions. Results: CDs with 0.4 mm WT (CD-0-0.4) exhibited higher swelling and erosion, lower adhesion, and faster disintegration, leading to a more immediate drug release, comparable to HGCs. A strong correlation was found between in vitro and in vivo disintegration behavior. Water sorption tests revealed lower moisture affinity for PVA CDs compared to HGC. Stability studies showed that CD-0-0.4 retained its physical and chemical properties. Instead, CDs with 0.9 mm WT (CD-0-0.9) were sensitive to storage, particularly under accelerated aging, which affected their integrity and release profile. Conclusions: These findings highlight the potential of PVA-CDs, especially the 0.4 mm design, as a promising and stable alternative for compounding pharmacy applications, offering an effective platform for personalized oral drug delivery.
Keywords: 3D printing; capsular devices; fused deposition modeling; in vivo studies; magistral compounding; stability studies.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures










Similar articles
-
Three-Dimensional Printing of PVA Capsular Devices for Applications in Compounding Pharmacy: Effect of Design Parameters on Pharmaceutical Performance.Pharmaceutics. 2024 Aug 15;16(8):1069. doi: 10.3390/pharmaceutics16081069. Pharmaceutics. 2024. PMID: 39204414 Free PMC article.
-
3D printing of PVA capsular devices for modified drug delivery: design and in vitro dissolution studies.Drug Dev Ind Pharm. 2020 Sep;46(9):1416-1426. doi: 10.1080/03639045.2020.1791166. Epub 2020 Jul 10. Drug Dev Ind Pharm. 2020. PMID: 32619117
-
Design and production of 3D printed oral capsular devices for the modified release of urea in ruminants.Int J Pharm. 2022 Nov 25;628:122353. doi: 10.1016/j.ijpharm.2022.122353. Epub 2022 Oct 29. Int J Pharm. 2022. PMID: 36349612
-
Extrusion-based 3D printing for development of complex capsular systems for advanced drug delivery.Int J Pharm. 2024 Sep 30;663:124550. doi: 10.1016/j.ijpharm.2024.124550. Epub 2024 Aug 3. Int J Pharm. 2024. PMID: 39103062 Review.
-
Polyvinyl Alcohol, a Versatile Excipient for Pharmaceutical 3D Printing.Polymers (Basel). 2024 Feb 14;16(4):517. doi: 10.3390/polym16040517. Polymers (Basel). 2024. PMID: 38399895 Free PMC article. Review.
References
-
- Fernandes A.I., Pereira G.G., Pinto J.F. Digital Compounding in Pharmacies: A Pilot Stability Study. Med. Sci. Forum. 2023;22:9. doi: 10.3390/msf2023022009. - DOI
-
- Topelius N.S., Shokraneh F., Bahman M., Lahtinen J., Hassinen N., Airaksinen S., Verma S., Hrizanovska L., Lass J., Paaver U., et al. Automated Non-Sterile Pharmacy Compounding: A Multi-Site Study in European Hospital and Community Pharmacies with Pediatric Immediate Release Propranolol Hydrochloride Tablets. Pharmaceutics. 2024;16:678. doi: 10.3390/pharmaceutics16050678. - DOI - PMC - PubMed
-
- Bianchi M., Pegoretti A., Fredi G. An Overview of Poly(vinyl alcohol) and Poly(vinyl pyrrolidone) in Pharmaceutical Additive Manufacturing. John Wiley and Sons Ltd.; Hoboken, NJ, USA: 2023. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

