Neutrophil-Camouflaged Stealth Liposomes for Photothermal-Induced Tumor Immunotherapy Through Intratumoral Bacterial Activation
- PMID: 40430905
- PMCID: PMC12115177
- DOI: 10.3390/pharmaceutics17050614
Neutrophil-Camouflaged Stealth Liposomes for Photothermal-Induced Tumor Immunotherapy Through Intratumoral Bacterial Activation
Abstract
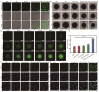
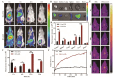
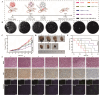
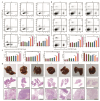
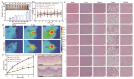
Objective: F. nucleatum, a tumor-resident bacterium colonizing breast cancer (BC), results in an immunosuppressive microenvironment and facilitates tumor growth and metastasis. This study aimed to develop a neutrophil-based liposome delivery system designed for dual-targeted elimination of tumor cells and F. nucleatum, while simultaneously upregulating pathogen-associated molecular patterns and damage-associated molecular patterns to potentiate tumor immunotherapy. Methods: The liposomes (PD/GA-LPs) loaded with the perylene diimide complex (PD) and gambogic acid (GA) were fabricated via the extrusion method. Subsequently, comprehensive evaluations including physicochemical characteristics, antibacterial activity, antitumor effect, and immunomodulatory effect evaluation were systematically conducted to validate the feasibility of this delivery system. Results: The resulting PD/GA-LPs exhibited a dynamic size (121.3 nm, zeta potential -44.1 mV) and a high encapsulation efficiency of approximately 78.1% (PD) and 91.8% (GA). In addition, the optimized PD/GA-LPs exhibited excellent photothermal performance and antibacterial efficacy. In vitro cellular experiments revealed that PD/GA-LPs exhibited enhanced internalization by neutrophils, followed by extracellular trap-mediated release, ultimately significantly inhibiting tumor cell proliferation and inducing immunogenic cell death. During in vivo treatment, PD/GA-LPs exhibited targeted tumor accumulation, where F. nucleatum-driven PD reduction activated near-infrared-responsive photothermal ablation. When combined with GA, this delivery system effectively eliminated tumor cells and F. nucleatum, while facilitating the subsequent T-cell infiltration. Conclusions: This strategy amplified the antitumor immune response, thus leading to effective treatment of BC and prevention of metastasis. In summary, this approach, grounded in the distinct microecology of tumor and normal tissues, offers novel insights into the development of precise and potent immunotherapies for BC.
Keywords: breast cancer; intratumor bacteria; neutrophil; photoimmunotherapy; tumor immune microenvironment.
Conflict of interest statement
The authors declare no conflicts of interest. All authors have read and agreed to the published version of the manuscript.
Figures




Similar articles
-
Functionalized biomimetic nanoparticles combining programmed death-1/programmed death-ligand 1 blockade with photothermal ablation for enhanced colorectal cancer immunotherapy.Acta Biomater. 2023 Feb;157:451-466. doi: 10.1016/j.actbio.2022.11.043. Epub 2022 Nov 25. Acta Biomater. 2023. PMID: 36442821
-
Biomimetic Nanomodulators With Synergism of Photothermal Therapy and Vessel Normalization for Boosting Potent Anticancer Immunity.Adv Mater. 2024 Oct;36(40):e2408511. doi: 10.1002/adma.202408511. Epub 2024 Aug 23. Adv Mater. 2024. PMID: 39180264
-
Transforming Intratumor Bacteria into Immunopotentiators to Reverse Cold Tumors for Enhanced Immuno-chemodynamic Therapy of Triple-Negative Breast Cancer.J Am Chem Soc. 2023 Dec 6;145(48):26296-26307. doi: 10.1021/jacs.3c09472. Epub 2023 Nov 21. J Am Chem Soc. 2023. PMID: 37987621
-
FAP-targeted radioligand therapy with 68Ga/177Lu-DOTA-2P(FAPI)2 enhance immunogenicity and synergize with PD-L1 inhibitors for improved antitumor efficacy.J Immunother Cancer. 2025 Jan 11;13(1):e010212. doi: 10.1136/jitc-2024-010212. J Immunother Cancer. 2025. PMID: 39800373 Free PMC article.
-
Advancements in Stimulus-Responsive Co-Delivery Nanocarriers for Enhanced Cancer Immunotherapy.Int J Nanomedicine. 2024 Apr 8;19:3387-3404. doi: 10.2147/IJN.S454004. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38617801 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

