LcTprxII Overexpression Enhances Physiological and Biochemical Effects in Maize Under Alkaline (Na2CO3) Stress
- PMID: 40431032
- PMCID: PMC12114990
- DOI: 10.3390/plants14101467
LcTprxII Overexpression Enhances Physiological and Biochemical Effects in Maize Under Alkaline (Na2CO3) Stress
Abstract
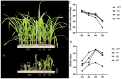
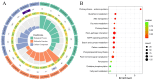
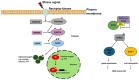
Alkaline stress limits crop productivity by causing osmotic and oxidative damage. This study investigated the new gene LcTprxII, a type II peroxiredoxin encoded by Leymus chinensis, and its role in enhancing alkaline stress tolerance in transgenic maize. The gene was cloned, overexpressed, and characterized using RT-PCR, phylogenetic analysis, and motif identification. Transgenic maize lines were generated via Agrobacterium-mediated transformation and subjected to physiological, biochemical, and transcriptomic analyses under alkaline stress. Under alkaline stress, the results revealed that LcTprxII overexpression significantly preserved chlorophyll content, mitigated oxidative damage, and maintained growth compared to wild-type plants, as evidenced by elevated activities of antioxidant enzymes (APX, CAT, SOD, and POD) and reduced malondialdehyde (MDA) content. Transcriptomic profiling identified 3733 differentially expressed genes and the upregulation of ABA and MAPK signaling pathways, highlighting the role of these genes in stress signaling and metabolic adaptation. Hormonal analysis indicated reduced ABA and increased GA levels in the transgenic lines. This study identified WRKY, bHLH, and MYB transcription factors as key regulators activated under alkaline stress, contributing to transcriptional regulation in transgenic maize. Field trials confirmed the agronomic potential of LcTprxII-overexpressing maize, with yield maintained under alkaline conditions. The present study revealed that LcTprxII enhances antioxidant defenses and stress signaling, which trigger tolerance to abiotic stress. Future studies should explore the long-term effects on growth, yield, and molecular interactions under diverse environmental conditions.
Keywords: LcTprxII; alkaline stress; hormone regulation; transcriptome; type II peroxiredoxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


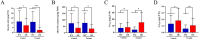

References
-
- Wang Y., Ma H., Liu G., Xu C., Zhang D., Ban Q. Analysis of Gene Expression Profile of Limonium bicolor under NaHCO3 Stress Using cDNA Microarray. Plant Mol. Biol. Rep. 2008;26:241–254. doi: 10.1007/s11105-008-0037-4. - DOI
-
- Yu Y., Duan X., Ding X., Chen C., Zhu D., Yin K., Cao L., Song X., Zhu P., Li Q., et al. A novel AP2/ERF family transcription factor from Glycine soja, GsERF71, is a DNA binding protein that positively regulates alkaline stress tolerance in Arabidopsis. Plant Mol. Biol. 2017;94:509–530. doi: 10.1007/s11103-017-0623-7. - DOI - PubMed
-
- Sun X., Yang S., Sun M., Wang S., Ding X., Zhu D., Ji W., Cai H., Zhao C., Wang X., et al. A novel Glycine soja cysteine proteinase inhibitor GsCPI14, interacting with the calcium/calmodulin-binding receptor-like kinase GsCBRLK, regulated plant tolerance to alkali stress. Plant Mol. Biol. 2014;85:33–48. doi: 10.1007/s11103-013-0167-4. - DOI - PubMed
-
- Yang C.W., Xu H.H., Wang L.L., Liu J., Shi D.C., Wang D.L. Comparative effects of salt-stress and alkali-stress on the growth, photosynthesis, solute accumulation, and ion balance of barley plants. Photosynthetica. 2009;47:79–86. doi: 10.1007/s11099-009-0013-8. - DOI
-
- Wang Z., Zhu S., Yu R. Saline Soil in China. Science Press Beijing; Beijing, China: 1993. (In Chinese)
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

