Some Insights into the Inventiveness of Dinoflagellates: Coming Back to the Cell Biology of These Protists
- PMID: 40431143
- PMCID: PMC12114471
- DOI: 10.3390/microorganisms13050969
Some Insights into the Inventiveness of Dinoflagellates: Coming Back to the Cell Biology of These Protists
Abstract
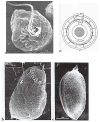
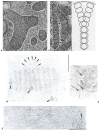
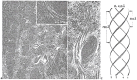
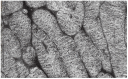
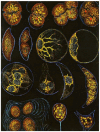
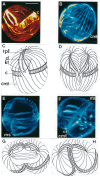
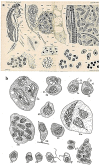
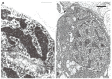
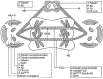
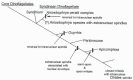
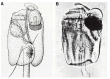
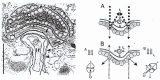
In this review dedicated to the great protistologist Edouard Chatton (1883-1947), I wanted to highlight the originality and remarkable diversity of some dinoflagellate protists through the lens of cell biology. Their fossilized traces date back to more than 538 million years (Phanerozoic eon). However, they may be much older because acritarchs from the (Meso) Proterozoic era (1500 million years ago) could be their most primitive ancestors. Here, I described several representative examples of the various lifestyles of free-living (the autotrophic thecate Prorocentrum micans Ehrenberg and the heterotrophic athecate Noctiluca scintillans McCartney and other "pseudo-noctilucidae", as well as the thecate Crypthecodinium cohnii Biecheler) and of parasitic dinoflagellates (the mixotroph Syndinium Chatton). Then, I compared the different dinoflagellate mitotic systems and reported observations on the eyespot (ocelloid), an organelle that is present in the binucleated Glenodinium foliaceum Stein and in some Warnowiidae dinoflagellates and can be considered an evolutionary marker. The diversity and innovations observed in mitosis, meiosis, reproduction, sexuality, cell cycle, locomotion, and nutrition allow us to affirm that dinoflagellates are among the most innovative unicells in the Kingdom Protista.
Keywords: cell biology; dinoflagellates; evolution; innovative features.
Conflict of interest statement
The author declares no conflicts of interest.
Figures












Similar articles
-
Edouard Chatton (1883-1947) and the dinoflagellate protists: concepts and models.Int Microbiol. 2006 Sep;9(3):173-7. Int Microbiol. 2006. PMID: 17061207
-
Major transitions in dinoflagellate evolution unveiled by phylotranscriptomics.Proc Natl Acad Sci U S A. 2017 Jan 10;114(2):E171-E180. doi: 10.1073/pnas.1614842114. Epub 2016 Dec 27. Proc Natl Acad Sci U S A. 2017. PMID: 28028238 Free PMC article.
-
Microtubule organization during the cell cycle of the primitive eukaryote dinoflagellate Crypthecodinium cohnii.J Cell Sci. 1993 Mar;104 ( Pt 3):639-51. doi: 10.1242/jcs.104.3.639. J Cell Sci. 1993. PMID: 8314867
-
[Cytoskeletal actin and its associated proteins. Some examples in Protista].Parasite. 1998 Jun;5(2):107-17. doi: 10.1051/parasite/1998052107. Parasite. 1998. PMID: 9754306 Review. French.
-
Chromosomes of Protists: The crucible of evolution.Int Microbiol. 2015 Dec;18(4):209-16. doi: 10.2436/20.1501.01.252. Int Microbiol. 2015. PMID: 27611673 Review.
References
-
- Chatton E. Les Péridiniens Parasites: Morphologie, Reproduction, Ethologie. Librairie, H. Le Soudier; Paris, France: 1920. pp. 1–475.
-
- Chatton E. Titres et Travaux Scientifiques (1906–1937) Imp-Editor Sottano; Sète, France: 1938. pp. 1–405.
-
- Soyer-Gobillard M.-O., Schrével J. The Discoveries and Artistic Talents of Edouard Chatton and André Lwoff, Famous Biologists. 1st ed. Cambridge Scholars Publishing; Cambridge, UK: 2021. pp. 1–228.
-
- Chatton E. Pansporella perplexa. Ann. Sci. Nat. Zool. 1925;8:1–85.
-
- Taylor F.J.R. Phylum Dinoflagellata. In: Margulis L., Corliss J., Melkonian M., Chapman D.J., McKhan H.I., editors. Handbook of Protoctista. 1st ed. Jones and Bartlett Publishers; Boston, MA, USA: 1990. pp. 419–437.
Publication types
LinkOut - more resources
Full Text Sources

