Infection with the Endonuclear Symbiotic Bacterium Holospora obtusa Reversibly Alters Surface Antigen Expression of the Host Paramecium caudatum
- PMID: 40431163
- PMCID: PMC12113882
- DOI: 10.3390/microorganisms13050991
Infection with the Endonuclear Symbiotic Bacterium Holospora obtusa Reversibly Alters Surface Antigen Expression of the Host Paramecium caudatum
Abstract
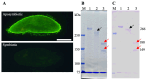
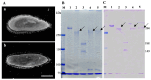
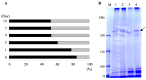
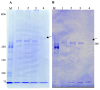
It is known that the ciliate Paramecium cell surface including cilia is completely covered by high-molecular-mass GPI-anchored proteins named surface antigens (SAgs). However, their functions are not well understood. It was found that ciliate Paramecium caudatum reversibly changes its SAgs depending on the absence or presence of the endonuclear symbiotic bacterium Holospora obtusa in the macronucleus. Immunofluorescence microscopy with a monoclonal antibody produced SAg of the H. obtusa-free P. caudatum strain RB-1-labeled cell surface of the H. obtusa-free P. caudatum RB-1 cell but not the H. obtusa-bearing RB-1 cell. When this antibody was added to the living P. caudatum RB-1 cells, only H. obtusa-free cells were immobilized. An immunoblot with SAgs extracted from Paramecium via cold salt/ethanol treatment showed approximately 266-kDa SAgs in the extract from H. obtusa-free cells and 188 and 149-kDa SAgs in the extract from H. obtusa-bearing cells. H. obtusa-free RB-1 cells produced from H. obtusa-bearing cells via treatment with penicillin-G-potassium re-expressed 266-kDa SAg, while the 188 and 149-kDa SAgs disappeared. This phenotypic change in the SAgs was not induced by degrees of starvation or temperature shifts. These results definitively show that Paramecium SAgs have functions related to bacterial infection.
Keywords: GPI-anchored surface protein; Holospora obtusa; Paramecium caudatum; bacterial infection; endonuclear symbiotic bacteria; endosymbiosis; immobilization antigen; phenotypic change in the host; surface antigen.
Conflict of interest statement
The author declares no conflicts of interest.
Figures

Similar articles
-
Paramecium caudatum acquires heat-shock resistance in ciliary movement by infection with the endonuclear symbiotic bacterium Holospora obtusa.FEMS Microbiol Lett. 2005 Feb 1;243(1):101-5. doi: 10.1016/j.femsle.2004.11.053. FEMS Microbiol Lett. 2005. PMID: 15668007
-
A 63-kDa Periplasmic Protein of the Endonuclear Symbiotic Bacterium Holospora obtusa Secreted to the Outside of the Bacterium during the Early Infection Process Binds Weakly to the Macronuclear DNA of the Host Paramecium caudatum.Microorganisms. 2023 Jan 7;11(1):155. doi: 10.3390/microorganisms11010155. Microorganisms. 2023. PMID: 36677447 Free PMC article.
-
Draft genome sequences of three Holospora species (Holospora obtusa, Holospora undulata, and Holospora elegans), endonuclear symbiotic bacteria of the ciliate Paramecium caudatum.FEMS Microbiol Lett. 2014 Oct;359(1):16-8. doi: 10.1111/1574-6968.12577. FEMS Microbiol Lett. 2014. PMID: 25115770
-
[The route of a bacterium Holospora in the cell of Paramecium (Ciliophora, Protista) from phagosome to the nucleus].Tsitologiia. 2002;44(11):1089-96. Tsitologiia. 2002. PMID: 12561729 Review. Russian.
-
Mechanisms for establishing primary and secondary endosymbiosis in Paramecium.J Eukaryot Microbiol. 2022 Sep;69(5):e12901. doi: 10.1111/jeu.12901. Epub 2022 Mar 19. J Eukaryot Microbiol. 2022. PMID: 35243727 Review.
References
-
- Preer J.R., Jr. Genetics of the protozoa. In: Chen T.T., editor. Research in Protozoology 3. Pergamon; Oxford, UK: 1968. pp. 129–278.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

