A Quaternary Sedimentary Ancient DNA (sed aDNA) Record of Fungal-Terrestrial Ecosystem Dynamics in a Tropical Biodiversity Hotspot (Lake Towuti, Sulawesi, Indonesia)
- PMID: 40431178
- PMCID: PMC12113726
- DOI: 10.3390/microorganisms13051005
A Quaternary Sedimentary Ancient DNA (sed aDNA) Record of Fungal-Terrestrial Ecosystem Dynamics in a Tropical Biodiversity Hotspot (Lake Towuti, Sulawesi, Indonesia)
Abstract
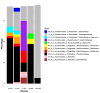
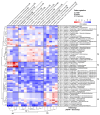
Short-term observations suggest that environmental changes affect the diversity and composition of soil fungi, significantly influencing forest resilience, plant diversity, and soil processes. However, time-series experiments should be supplemented with geobiological archives to capture the long-term effects of environmental changes on fungi-soil-plant interactions, particularly in undersampled, floristically diverse tropical forests. We recently conducted trnL-P6 amplicon sequencing to generate a sedimentary ancient DNA (sedaDNA) record of the regional catchment vegetation of the tropical waterbody Lake Towuti (Sulawesi, Indonesia), spanning over one million years (Myr) of the lake's developmental history. In this study, we performed 18SV9 amplicon sequencing to create a parallel paleofungal record to (a) infer the composition, origins, and functional guilds of paleofungal community members and (b) determine the extent to which downcore changes in fungal community composition reflect the late Pleistocene evolution of the Lake Towuti catchment. We identified at least 52 members of Ascomycota (predominantly Dothiodeomycetes, Eurotiomycetes, and Leotiomycetes) and 12 members of Basidiomycota (primarily Agaricales and Polyporales). Spearman correlation analysis of the relative changes in fungal community composition, geochemical parameters, and paleovegetation assemblages revealed that the overwhelming majority consisted of soil organic matter and wood-decaying saprobes, except for a necrotrophic phytopathogenic association between Mycosphaerellaceae (Cadophora) and wetland herbs (Alocasia) in more-than-1-Myr-old silts and peats deposited in a pre-lake landscape, dominated by small rivers, wetlands, and peat swamps. During the lacustrine stage, vegetation that used to grow on ultramafic catchment soils during extended periods of inferred drying showed associations with dark septate endophytes (Ploettnerulaceae and Didymellaceae) that can produce large quantities of siderophores to solubilize mineral-bound ferrous iron, releasing bioavailable ferrous iron needed for several processes in plants, including photosynthesis. Our study showed that sedaDNA metabarcoding paired with the analysis of geochemical parameters yielded plausible insights into fungal-plant-soil interactions, and inferred changes in the paleohydrology and catchment evolution of tropical Lake Towuti, spanning more than one Myr of deposition.
Keywords: Lake Towuti; felsic; fungi; quaternary; sedaDNA; tropics; ultramafic.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
A 1 Ma sedimentary ancient DNA (sedaDNA) record of catchment vegetation changes and the developmental history of tropical Lake Towuti (Sulawesi, Indonesia).Geobiology. 2024 May-Jun;22(3):e12599. doi: 10.1111/gbi.12599. Geobiology. 2024. PMID: 38745401
-
Reconstructing long-term human impacts on plant communities: an ecological approach based on lake sediment DNA.Mol Ecol. 2015 Apr;24(7):1485-98. doi: 10.1111/mec.13136. Epub 2015 Mar 23. Mol Ecol. 2015. PMID: 25735209
-
Sedimentary Ancient DNA (sedaDNA) Reveals Fungal Diversity and Environmental Drivers of Community Changes throughout the Holocene in the Present Boreal Lake Lielais Svētiņu (Eastern Latvia).Microorganisms. 2021 Mar 31;9(4):719. doi: 10.3390/microorganisms9040719. Microorganisms. 2021. PMID: 33807307 Free PMC article.
-
Use of ancient sedimentary DNA as a novel conservation tool for high-altitude tropical biodiversity.Conserv Biol. 2014 Apr;28(2):446-55. doi: 10.1111/cobi.12195. Epub 2013 Dec 26. Conserv Biol. 2014. PMID: 24372820
-
Ectomycorrhizal associations in the tropics - biogeography, diversity patterns and ecosystem roles.New Phytol. 2018 Dec;220(4):1076-1091. doi: 10.1111/nph.15151. Epub 2018 Apr 24. New Phytol. 2018. PMID: 29689121 Review.
Cited by
-
The Deep Subsurface Biosphere and its Substrates Along a One-Million-Year Ferruginous Lake Archive.Microb Ecol. 2025 Jun 3;88(1):58. doi: 10.1007/s00248-025-02559-4. Microb Ecol. 2025. PMID: 40461733 Free PMC article.
References
-
- Amir H., Jourand P., Cavaloc Y., Ducousso M. Role of Mycorrhizal Fungi in the Alleviation of Heavy Metal Toxicity in Plants. In: Solaiman Z., Abbott L., Varma A., editors. Mycorrhizal Fungi: Use in Sustainable Agriculture and Land Restoration. Volume 41. Springer; Berlin/Heidelberg, Germany: 2014. Soil Biology. - DOI
-
- Dighton J. Fungi in Ecosystem Processes. 2nd ed. CRC Press; Boca Raton, FL, USA: 2016. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

