Reducing Functional Domain of Histatin 5 Improves Antifungal Activity and Prevents Proteolytic Degradation
- PMID: 40431266
- PMCID: PMC12113730
- DOI: 10.3390/microorganisms13051091
Reducing Functional Domain of Histatin 5 Improves Antifungal Activity and Prevents Proteolytic Degradation
Abstract
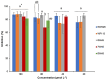
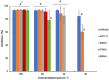
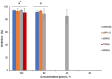
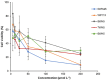
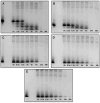
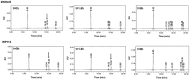
Histatin 5 (Hst5) is an antifungal peptide (AFP) naturally produced by parotid glands with strong activity against Candida albicans. One of its mechanisms of action is the generation of reactive oxygen species (ROS) inside the C. albicans cells. Despite being an important peptide for the human innate immune response, its activity is reduced or inactivated by proteolytic degradation caused by salivary enzymes. To overcome this barrier, we used solid phase peptide synthesis (SPPS) to modify the Hst5 amino acid sequence improving its antifungal action and minimizing its degradation. We synthesized five peptides, three of which were based on the Hst5 functional domain. We determined that the smallest peptides (8WH5, 7WH5 and 6WH5) demonstrated the greatest antifungal action against C. albicans, including one fluconazole-resistant strain. Besides that, cationic-PAGE and HPLC assays showed that the degradation in saliva was slower for the smaller peptides than for 0WHst5 and WP113. Furthermore, 8WH5, 7WH5 and 6WH5 were found in the samples even after 8 h in whole saliva, while 0WHst5 and WP113 completely disappear after 1.5 h. Finally, we found that the smaller peptides were less fragmented than the 0WHst5 and WP113, so they were the smallest fragments of Hst5 to preserve its antifungal action with reduced degradation in whole saliva. Thus, they can be considered promising molecules for the treatment of C. albicans in the oral cavity.
Keywords: P-113; SPPS; antifungal peptides; degradation; histatin 5; peptides; proteolysis; saliva.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

