Periplasmic Protein Mobility for Extracellular Electron Transport in Shewanella oneidensis
- PMID: 40431315
- PMCID: PMC12114092
- DOI: 10.3390/microorganisms13051144
Periplasmic Protein Mobility for Extracellular Electron Transport in Shewanella oneidensis
Abstract
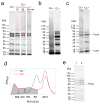
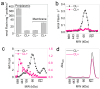
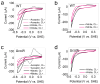
Extracellular electron transport (EET) supports the survival of specific microorganisms on the Earth's surface by facilitating microbial respiration with diverse electron acceptors. A key aspect of EET is the organization of electron relays, i.e., multi-heme c-type cytochromes (MHCs), within the periplasmic space of microbial cells. In this study, we investigated the mobility of periplasmic electron relays in Shewanella oneidensis MR-1, a model strain capable of EET, using in vivo protein crosslinking to the MHCs. First, we established that crosslinking efficiency correlates with the spatial proximity and diffusion coefficient of protein molecules through in vitro tests. Based on these findings, we identified distinct molecular behaviors of periplasmic MHCs, showing that the tetraheme flavocytochrome FccA, which also serves as a periplasmic fumarate reductase, forms protein complexes with limited motility, while the small tetraheme c-type cytochrome CctA remains discrete and mobile. Both MHCs contribute to EET for bioelectrochemical nitrate and nitrite reduction. These findings reveal dual mechanisms for organizing periplasmic electron relays in EET, advancing our understanding of microbial extracellular respiration.
Keywords: bioelectrochemistry; c-type cytochrome; crosslinking; extracellular electron transport; formaldehyde; microbial respiration.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Secreted Flavin Cofactors for Anaerobic Respiration of Fumarate and Urocanate by Shewanella oneidensis: Cost and Role.Appl Environ Microbiol. 2019 Aug 1;85(16):e00852-19. doi: 10.1128/AEM.00852-19. Print 2019 Aug 15. Appl Environ Microbiol. 2019. PMID: 31175188 Free PMC article.
-
Promoting Extracellular Electron Transfer of Shewanella oneidensis MR-1 by Optimizing the Periplasmic Cytochrome c Network.Front Microbiol. 2021 Oct 5;12:727709. doi: 10.3389/fmicb.2021.727709. eCollection 2021. Front Microbiol. 2021. PMID: 34675900 Free PMC article.
-
Periplasmic electron transfer via the c-type cytochromes MtrA and FccA of Shewanella oneidensis MR-1.Appl Environ Microbiol. 2009 Dec;75(24):7789-96. doi: 10.1128/AEM.01834-09. Epub 2009 Oct 16. Appl Environ Microbiol. 2009. PMID: 19837833 Free PMC article.
-
The membrane-bound tetrahaem c-type cytochrome CymA interacts directly with the soluble fumarate reductase in Shewanella.Biochem Soc Trans. 2002 Aug;30(4):658-62. doi: 10.1042/bst0300658. Biochem Soc Trans. 2002. PMID: 12196158 Review.
-
Engineering S. oneidensis for Performance Improvement of Microbial Fuel Cell-a Mini Review.Appl Biochem Biotechnol. 2021 Apr;193(4):1170-1186. doi: 10.1007/s12010-020-03469-6. Epub 2020 Nov 17. Appl Biochem Biotechnol. 2021. PMID: 33200267 Review.
References
-
- Madigan M.T., Bender K.S., Buckley D.H., Sattley W.M., Stahl D.A. Brock Biology of Microorganisms. 15th Global ed. Pearson Education Limited; New York, NY, USA: 2019. Metabolic Diversity of Microorganisms; pp. 428–486.
Grants and funding
LinkOut - more resources
Full Text Sources

