3,3'-Diindolylmethane Ameliorates Metabolism Dysfunction-Associated Fatty Liver Disease via AhR/p38 MAPK Signaling
- PMID: 40431421
- PMCID: PMC12113855
- DOI: 10.3390/nu17101681
3,3'-Diindolylmethane Ameliorates Metabolism Dysfunction-Associated Fatty Liver Disease via AhR/p38 MAPK Signaling
Abstract
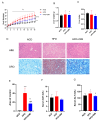
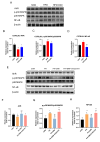
Background/Objectives: Metabolic dysfunction-associated fatty liver disease (MAFLD) is a chronic hepatic condition marked by lipid buildup, lipotoxicity, and inflammation. Prior research indicates that 3,3'-Diindolemethane (DIM), a natural indole-type phytochemical that is abundant in brassicaceae vegetables, has been reported to reduce body weight and improve lipid metabolism in mice subjected to a high-fat diet (HFD). The aryl hydrocarbon receptor (AhR), a nuclear receptor implicated in lipid metabolism and immune regulation, serves as a functional receptor for DIM. However, the underlying signaling pathways that regulate MAFLD remain elusive. Our objective is to ascertain the beneficial impact of DIM on MAFLD and the associated mechanisms. Methods: Hematoxylin and eosin staining, together with Oil Red O staining, were utilized to assess the pathological changes and lipid deposition in the liver. Biochemical analysis was employed to measure levels of triglyceride (TG), total cholesterol (TC), free fatty acid (FFA), aspartate transaminase (AST), alanine transaminase (ALT), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C). The cell survival rate of HepG2 cells treated with palmitic acid (PA) and DIM was assessed using the CCK-8 assay. Flow cytometry was employed to measure the fluorescence intensity emitted by lipid droplets within cells. Western blotting analysis was performed to assess AhR pathway and fatty acid transporter expression levels in hepatic tissue. Results: Our results showed that DIM significantly attenuated body weight gain and hepatic injury brought on by HFD, decreased lipid droplet accumulation in HepG2 cells, and effectively suppressed the phosphorylation of p38 MAPK and the protein expression levels of fatty acid transporters CD36 and FATP4. Conclusions: DIM reduced lipid accumulation by activating AhR and suppressing p38 MAPK phosphorylation, thereby inhibiting fatty acid transport and inflammatory responses. These findings suggest that DIM may represent a promising therapeutic candidate for MAFLD, warranting further exploration for clinical applications.
Keywords: 3,3′-Diindolylmethane; CD36; MAFLD; aryl hydrocarbon receptor; p38 MAPK.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M., Zelber-Sagi S., Wai-Sun Wong V., Dufour J.F., Schattenberg J.M., et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. - DOI - PubMed
-
- Li J., Zou B., Yeo Y.H., Feng Y., Xie X., Lee D.H., Fujii H., Wu Y., Kam L.Y., Ji F., et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2019;4:389–398. doi: 10.1016/S2468-1253(19)30039-1. - DOI - PubMed
-
- Ji L., Li Q., He Y., Zhang X., Zhou Z., Gao Y., Fang M., Yu Z., Rodrigues R.M., Gao Y., et al. Therapeutic potential of traditional Chinese medicine for the treatment of NAFLD: A promising drug Potentilla discolor Bunge. Acta Pharm. Sin. B. 2022;12:3529–3547. doi: 10.1016/j.apsb.2022.05.001. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

