Comparative Examination of Feline Coronavirus and Canine Coronavirus Effects on Extracellular Vesicles Acquired from A-72 Canine Fibrosarcoma Cell Line
- PMID: 40431570
- PMCID: PMC12115506
- DOI: 10.3390/vetsci12050477
Comparative Examination of Feline Coronavirus and Canine Coronavirus Effects on Extracellular Vesicles Acquired from A-72 Canine Fibrosarcoma Cell Line
Abstract
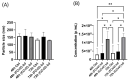
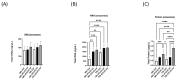
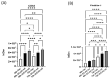
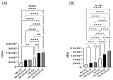
Introduction: Coronavirus (CoV) is an extremely contagious, enveloped positive-single-stranded RNA virus, which has become a global pandemic that causes several illnesses in humans and animals. Hence, it is necessary to investigate viral-induced reactions across diverse hosts. Herein, we propose utilizing naturally secreted extracellular vesicles (EVs), mainly focusing on exosomes to examine virus-host responses following CoV infection. Exosomes are small membrane-bound vesicles originating from the endosomal pathway, which play a pivotal role in intracellular communication and physiological and pathological processes. We suggested that CoV could impact EV formation, content, and diverse immune responses in vitro. Methods: In this study, we infected A-72, which is a canine fibroblast cell line, with a feline coronavirus (FCoV) and canine coronavirus (CCoV) independently in an exosome-free media at 0.001 multiplicity of infection (MOI), with incubation periods of 48 and 72 h. The cell viability was significantly downregulated with increased incubation time following FCoV and CCoV infection, which was identified by performing the 3-(4,5-dimethylthiazo-1-2yl)-2,5-diphenyltetrazolium bromide (MTT) assay. After the infection, EVs were isolated through ultracentrifugation, and the subsequent analysis involved quantifying and characterizing the purified EVs using various techniques. Results: NanoSight particle tracking analysis (NTA) verified that EV dimensions fell between 100 and 200 nm at both incubation periods. At both periods, total protein and RNA levels were significantly upregulated in A-72-derived EVs following FCoV and CCoV infections. However, total DNA levels were gradually upregulated with increased incubation time. Dot blot analysis indicated that the expression levels of ACE2, IL-1β, Flotillin-1, CD63, caspase-8, and Hsp90 were modified in A-72-derived EVs following both CoV infections. Conclusions: Our results indicated that FCoV and CCoV infections could modulate the EV production and content, which could play a role in the development of viral diseases. Investigating diverse animal CoV will provide in-depth insight into host exosome biology during CoV infection. Hence, our findings contribute to the comprehension and characterization of EVs in virus-host interactions during CoV infection.
Keywords: A-72 cells; canine coronavirus; exosomes; extracellular vesicles; feline coronavirus; immunomodulation; pathogenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Canine Coronavirus Infection Modulates the Biogenesis and Composition of Cell-Derived Extracellular Vesicles.Biomedicines. 2023 Mar 21;11(3):976. doi: 10.3390/biomedicines11030976. Biomedicines. 2023. PMID: 36979955 Free PMC article.
-
Feline coronavirus influences the biogenesis and composition of extracellular vesicles derived from CRFK cells.Front Vet Sci. 2024 Jul 18;11:1388438. doi: 10.3389/fvets.2024.1388438. eCollection 2024. Front Vet Sci. 2024. PMID: 39091390 Free PMC article.
-
Infection of cats with atypical feline coronaviruses harbouring a truncated form of the canine type I non-structural ORF3 gene.Infect Genet Evol. 2013 Dec;20:488-94. doi: 10.1016/j.meegid.2013.09.024. Epub 2013 Oct 9. Infect Genet Evol. 2013. PMID: 24121017 Free PMC article.
-
Extracellular Vesicles: Roles in Human Viral Infections, Immune-Diagnostic, and Therapeutic Applications.Pathogens. 2020 Dec 17;9(12):1056. doi: 10.3390/pathogens9121056. Pathogens. 2020. PMID: 33348699 Free PMC article. Review.
-
Extracellular Vesicles as a Translational Approach for the Treatment of COVID-19 Disease: An Updated Overview.Viruses. 2023 Sep 22;15(10):1976. doi: 10.3390/v15101976. Viruses. 2023. PMID: 37896755 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

