Dysfunctional Senescent Herpes Simplex Virus-Specific CD57+CD8+ T Cells Are Associated with Symptomatic Recurrent Ocular Herpes in Humans
- PMID: 40431618
- PMCID: PMC12115701
- DOI: 10.3390/v17050606
Dysfunctional Senescent Herpes Simplex Virus-Specific CD57+CD8+ T Cells Are Associated with Symptomatic Recurrent Ocular Herpes in Humans
Abstract
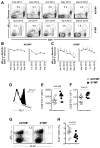
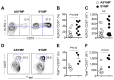
Herpes simplex virus (HSV)-specific CD8+ T cells protect mice from herpes infection and disease. However, the phenotype and function of HSV-specific CD8+ T cells that play a key role in the "natural" protection seen in HSV-1-seropositive healthy asymptomatic (ASYMP) patients (who have never had clinical herpes disease) remain to be determined. We previously reported that symptomatic (SYMP) patients (who have frequent bouts of recurrent ocular herpes disease) had more undifferentiated and dysfunctional HSV-specific CD8+ T cells. In contrast, healthy ASYMP individuals maintained a significantly higher proportion of differentiated polyfunctional CD8+ T cells. Here, we report that HSV-specific CD8+ T cells from 10 SYMP patients, but not HSV-specific CD8+ T cells from 10 ASYMP patients, have phenotypic and functional characteristics of cellular senescence, including: (i) high frequency of senescent (CD57+) and exhausted (PD-1+) CD8+ T cells; (ii) late terminally differentiated (KLRG1+), non-proliferating CD8+ T cells; (iii) HSV-specific CD8+ T cells which decreased in number over time and were not homeostatically maintained, as indicated by a reduction in the number of CD127+CD8+ T cells; (iv) loss of the co-stimulatory molecule CD28 on HSV-specific CD8+ T cells; and (v) decreased production of effector molecules (granzyme B and perforin) by HSV-specific CD8+ T cells. Our findings provide insights into the role of senescence in HSV-specific CD8+ T cells in susceptibility to recurrent herpes and have implications for T-cell-based immunotherapeutic strategies against recurrent herpes in humans.
Keywords: CD57; CD8+ T cells; cornea; ocular herpes; senescent; trigeminal ganglia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Human Asymptomatic Epitopes Identified from the Herpes Simplex Virus Tegument Protein VP13/14 (UL47) Preferentially Recall Polyfunctional Effector Memory CD44high CD62Llow CD8+ TEM Cells and Protect Humanized HLA-A*02:01 Transgenic Mice against Ocular Herpesvirus Infection.J Virol. 2017 Jan 3;91(2):e01793-16. doi: 10.1128/JVI.01793-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27847359 Free PMC article.
-
Phenotypic and functional characterization of herpes simplex virus glycoprotein B epitope-specific effector and memory CD8+ T cells from symptomatic and asymptomatic individuals with ocular herpes.J Virol. 2015 Apr;89(7):3776-92. doi: 10.1128/JVI.03419-14. Epub 2015 Jan 21. J Virol. 2015. PMID: 25609800 Free PMC article.
-
HLA-A02:01-restricted epitopes identified from the herpes simplex virus tegument protein VP11/12 preferentially recall polyfunctional effector memory CD8+ T cells from seropositive asymptomatic individuals and protect humanized HLA-A*02:01 transgenic mice against ocular herpes.J Immunol. 2015 Mar 1;194(5):2232-48. doi: 10.4049/jimmunol.1402606. Epub 2015 Jan 23. J Immunol. 2015. PMID: 25617474 Free PMC article.
-
Oral antivirals for preventing recurrent herpes simplex keratitis in people with corneal grafts.Cochrane Database Syst Rev. 2016 Nov 30;11(11):CD007824. doi: 10.1002/14651858.CD007824.pub2. Cochrane Database Syst Rev. 2016. PMID: 27902849 Free PMC article.
-
Interventions for prevention of herpes simplex labialis (cold sores on the lips).Cochrane Database Syst Rev. 2015 Aug 7;2015(8):CD010095. doi: 10.1002/14651858.CD010095.pub2. Cochrane Database Syst Rev. 2015. PMID: 26252373 Free PMC article.
References
-
- Samandary S., Kridane-Miledi H., Sandoval J.S., Choudhury Z., Langa-Vives F., Spencer D., Chentoufi A.A., Lemonnier F.A., BenMohamed L. Associations of HLA-A, HLA-B and HLA-C Alleles Frequency with Prevalence of Herpes Simplex Virus Infections and Diseases Across Global Populations: Implication for the Development of an Universal CD8+ T-Cell Epitope-Based Vaccine. Hum. Immunol. 2014;75:715–729. doi: 10.1016/j.humimm.2014.04.016. - DOI - PMC - PubMed
-
- Zhang X., Dervillez X., Chentoufi A.A., Badakhshan T., Bettahi I., BenMohamed L. Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes: Importance of MyD88. J. Immunol. 2012;189:4496–4509. doi: 10.4049/jimmunol.1201121. - DOI - PMC - PubMed
-
- Chentoufi A.A., Dasgupta G., Christensen N.D., Hu J., Choudhury Z.S., Azeem A., Jester J.V., Nesburn A.B., Wechsler S.L., BenMohamed L. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J. Immunol. 2010;184:2561–2571. doi: 10.4049/jimmunol.0902322. - DOI - PMC - PubMed
-
- Chentoufi A.A., Zhang X., Lamberth K., Dasgupta G., Bettahi I., Nguyen A., Wu M., Zhu X., Mohebbi A., Buus S., et al. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J. Immunol. 2008;180:426–437. doi: 10.4049/jimmunol.180.1.426. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY019896, EY024618, AI150091, and AI143348, (to L.B.M.)/National Institutes of Health Research R01 Grants,The Discovery Center for Eye Research and by an RPB Unrestricted Grant.
- R01 EY019896/EY/NEI NIH HHS/United States
- R01 EY024618/EY/NEI NIH HHS/United States
- R01 AI150091/AI/NIAID NIH HHS/United States
- R01 AI143348/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

