SARS-CoV-2 Spike Protein and Long COVID-Part 2: Understanding the Impact of Spike Protein and Cellular Receptor Interactions on the Pathophysiology of Long COVID Syndrome
- PMID: 40431631
- PMCID: PMC12115913
- DOI: 10.3390/v17050619
SARS-CoV-2 Spike Protein and Long COVID-Part 2: Understanding the Impact of Spike Protein and Cellular Receptor Interactions on the Pathophysiology of Long COVID Syndrome
Abstract
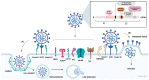
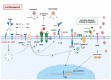
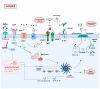
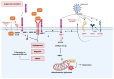
SARS-CoV-2 infection has had a significant impact on global health through both acute illness, referred to as coronavirus disease 2019 (COVID-19), and chronic conditions (long COVID or post-acute sequelae of COVID-19, PASC). Despite substantial advancements in preventing severe COVID-19 cases through vaccination, the rise in the prevalence of long COVID syndrome and a notable degree of genomic mutation, primarily in the S protein, underscores the necessity for a deeper understanding of the underlying pathophysiological mechanisms related to the S protein of SARS-CoV-2. In this review, the latest part of this series, we investigate the potential pathophysiological molecular mechanisms triggered by the interaction between the spike protein and cellular receptors. Therefore, this review aims to provide a differential and focused view on the mechanisms potentially activated by the binding of the spike protein to canonical and non-canonical receptors for SARS-CoV-2, together with their possible interactions and effects on the pathogenesis of long COVID.
Keywords: cellular receptors; interactions; spike protein.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Determinants of susceptibility to SARS-CoV-2 infection in murine ACE2.J Virol. 2025 Jun 17;99(6):e0054325. doi: 10.1128/jvi.00543-25. Epub 2025 May 12. J Virol. 2025. PMID: 40353671 Free PMC article.
-
SARS-CoV-2 Spike Protein and Long COVID-Part 1: Impact of Spike Protein in Pathophysiological Mechanisms of Long COVID Syndrome.Viruses. 2025 Apr 25;17(5):617. doi: 10.3390/v17050617. Viruses. 2025. PMID: 40431629 Free PMC article. Review.
-
The LDL receptor-related protein 1 (LRP1) facilitates ACE2-mediated endocytosis of SARS-CoV2 spike protein-containing pseudovirions.J Biol Chem. 2025 Jun;301(6):110227. doi: 10.1016/j.jbc.2025.110227. Epub 2025 May 9. J Biol Chem. 2025. PMID: 40349772 Free PMC article.
-
The effect of early COVID-19 treatment with convalescent plasma on antibody responses to SARS-CoV-2.Microbiol Spectr. 2025 Jul;13(7):e0300624. doi: 10.1128/spectrum.03006-24. Epub 2025 Jun 9. Microbiol Spectr. 2025. PMID: 40488473 Free PMC article. Clinical Trial.
-
Post-acute sequelae SARS-CoV-2 infection and neuropathic pain: a narrative review of the literature and future directions.Pain Manag. 2025 Jun;15(6):333-343. doi: 10.1080/17581869.2025.2501521. Epub 2025 May 14. Pain Manag. 2025. PMID: 40366711 Review.
References
-
- Cascella M., Rajnik M., Aleem A., Dulebohn S.C., Di Napoli R. StatPearls. StatPearls Publishing LLC.; Treasure Island, FL, USA: 2024. Features, Evaluation, and Treatment of Coronavirus (COVID-19) - PubMed
-
- Pizzato M., Baraldi C., Boscato Sopetto G., Finozzi D., Gentile C., Gentile M.D., Marconi R., Paladino D., Raoss A., Riedmiller I., et al. SARS-CoV-2 and the Host Cell: A Tale of Interactions. Front. Virol. 2022;1:815388. doi: 10.3389/fviro.2021.815388. - DOI
-
- Fontes-Dantas F.L., Fernandes G.G., Gutman E.G., De Lima E.V., Antonio L.S., Hammerle M.B., Mota-Araujo H.P., Colodeti L.C., Araujo S.M.B., Froz G.M., et al. SARS-CoV-2 Spike protein induces TLR4-mediated long-term cognitive dysfunction recapitulating post-COVID-19 syndrome in mice. Cell Rep. 2023;42:112189. doi: 10.1016/j.celrep.2023.112189. - DOI - PMC - PubMed
-
- Kong W., Montano M., Corley M.J., Helmy E., Kobayashi H., Kinisu M., Suryawanshi R., Luo X., Royer L.A., Roan N.R., et al. Neuropilin-1 Mediates SARS-CoV-2 Infection of Astrocytes in Brain Organoids, Inducing Inflammation Leading to Dysfunction and Death of Neurons. mBio. 2022;13:e0230822. doi: 10.1128/mbio.02308-22. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

