Time-Frequency Analysis of Motor Imagery During Plantar and Dorsal Flexion Movements Using a Low-Cost Ankle Exoskeleton
- PMID: 40431780
- PMCID: PMC12115110
- DOI: 10.3390/s25102987
Time-Frequency Analysis of Motor Imagery During Plantar and Dorsal Flexion Movements Using a Low-Cost Ankle Exoskeleton
Abstract
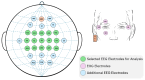
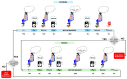
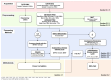
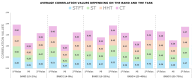
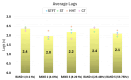
Sensor technology plays a fundamental role in neuro-motor rehabilitation by enabling precise movement analysis and control. This study explores the integration of brain-machine interfaces (BMIs) and wearable sensors to enhance motor recovery in individuals with neuro-motor impairments. Specifically, different time-frequency transforms are evaluated to analyze the correlation between electroencephalographic (EEG) activity and ankle position, measured by using inertial measurement units (IMUs). A low-cost ankle exoskeleton was designed to conduct the experimental trials. Six subjects performed plantar and dorsal flexion movements while the EEG and IMU signals were recorded. The correlation between brain activity and foot kinematics was analyzed using the Short-Time Fourier Transform (STFT), Stockwell (ST), Hilbert-Huang (HHT), and Chirplet (CT) methods. The 8-20 Hz frequency band exhibited the highest correlation values. For motor imagery classification, the STFT achieved the highest accuracy (92.9%) using an EEGNet-based classifier and a state-machine approach. This study presents a dual approach: the analysis of EEG-movement correlation in different cognitive states, and the systematic comparison of four time-frequency transforms for both correlation and classification performance. The results support the potential of combining EEG and IMU data for BMI applications and highlight the importance of cognitive state in motion analysis for accessible neurorehabilitation technologies.
Keywords: brain–machine interface (BMI); electroencephalography (EEG); inertial measurement units (IMUs); low-cost exoskeleton; motor imagery; neurorehabilitation; time-frequency transforms.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Ferrero L., Ortiz M., Quiles V., Iáñez E., Flores J.A., Azorín J.M. Brain symmetry analysis during the use of a BCI based on motor imagery for the control of a lower-limb exoskeleton. Symmetry. 2021;13:1746. doi: 10.3390/sym13091746. - DOI
-
- Shyam A.K.B., Wadhwani Investigation of the Impact of EEG Signal Processing Techniques on Classification Performance. In: Kumar Singh K., Singh S., Srivastava S., Bajpai M.K., editors. Proceedings of the Machine Vision and Augmented Intelligence; Patna, India. 24–25 November 2023; Singapore: Springer Nature; 2023. pp. 265–283.
MeSH terms
Grants and funding
- PID2021-124111OB-C31/MICIU /AEI/10.13039/501100011033 and by ERDF, EU
- PRE2022-103336/MICIU/AEI/10.13039 501100011033
- Valencian Graduate School and Research Network of Artificial Intelligence (ValgrAI), Generalitat Valenciana and European Union
- Project "Neurokit" funded by Centro Internacional para la Investigación del Envejecimiento de la Fundación de la Comunitat Valenciana (ICAR)
- 101118964/European Union's research and innovation programme under the Marie Skłodowska-Curie
LinkOut - more resources
Full Text Sources

