Host Immune Response to Bovine Viral Diarrhea Virus (BVDV): Insights and Strategies for Effective Vaccine Design
- PMID: 40432068
- PMCID: PMC12115442
- DOI: 10.3390/vaccines13050456
Host Immune Response to Bovine Viral Diarrhea Virus (BVDV): Insights and Strategies for Effective Vaccine Design
Abstract
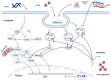
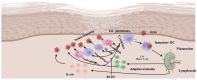
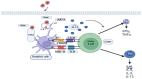
Bovine viral diarrhea (BVD) is caused by bovine viral diarrhea virus (BVDV), a member of the genus Pestivirus and in the family Flaviviridae. According to some studies, the disease incurs USD 1.5-2.5 billion per year and USD 0.50 to USD 687.80 per cow loss in beef and dairy farms, respectively. Using vaccines is among the strategies to prevent the disease. However, complete protection requires vaccines that target both the humoral and cellular immune responses of the adaptive immune system. A comprehensive literature review was made to provide insights into the interaction of BVDV with host immunity, vaccine applications, and the limitation of the currently available vaccines, as well as explore strategies used to advance the vaccines. BVDV causes immunosuppression by interfering with the innate and adaptive immune systems in a manner that is species and biotype-dependent. Interferon production, apoptosis, neutrophil activity, and antigen-processing and presenting cells are significantly affected during the viral infection. Despite maternal antibodies (MatAbs) being crucial to protect calves from early-age infection, a higher level of MatAbs are counterproductive during the immunization of calves. There are numerous inactivated or modified BVDV vaccines, most of which are made of cytopathic BVDV 1 and 2 and the BVDV 1a subgenotypes. Furthermore, subunit, marker, DNA and mRNA vaccines are made predominantly from E2, Erns, and NS3 proteins of the virus in combination with modern adjuvants, although the vaccines have not yet been licensed for use and are in the experimental stage. The existing BVDV vaccines target the humoral immune system, which never gives the full picture of protection without the involvement of the cell-mediated immune system. Several limitations were associated with conventional and next-generation vaccines that reduce BVDV vaccine efficiency. In general, providing complete protection against BVDV is very complex, which requires a multi-pronged approach to study factors affecting vaccine efficacy and strategies needed to improve vaccine efficacy and safety.
Keywords: BVD; BVDV; immunity; maternal antibody; vaccines.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Immunogenicity of an inactivated Chinese bovine viral diarrhea virus 1a (BVDV 1a) vaccine cross protects from BVDV 1b infection in young calves.Vet Immunol Immunopathol. 2014 Aug 15;160(3-4):288-92. doi: 10.1016/j.vetimm.2014.04.007. Epub 2014 Apr 29. Vet Immunol Immunopathol. 2014. PMID: 24880701
-
Mosaic Bovine Viral Diarrhea Virus Antigens Elicit Cross-Protective Immunity in Calves.Front Immunol. 2020 Nov 12;11:589537. doi: 10.3389/fimmu.2020.589537. eCollection 2020. Front Immunol. 2020. PMID: 33281819 Free PMC article.
-
Recombinant Erns-E2 protein vaccine formulated with MF59 and CPG-ODN promotes T cell immunity against bovine viral diarrhea virus infection.Vaccine. 2020 May 8;38(22):3881-3891. doi: 10.1016/j.vaccine.2020.03.020. Epub 2020 Apr 10. Vaccine. 2020. PMID: 32280039
-
Maternal antibody blocks humoral but not T cell responses to BVDV.Biologicals. 2003 Jun;31(2):123-5. doi: 10.1016/s1045-1056(03)00027-7. Biologicals. 2003. PMID: 12770543 Review.
-
Impact of species and subgenotypes of bovine viral diarrhea virus on control by vaccination.Anim Health Res Rev. 2015 Jun;16(1):40-54. doi: 10.1017/S1466252315000079. Anim Health Res Rev. 2015. PMID: 26050571 Review.
References
-
- Szabára Á., Ózsvári L. Challenges for the Agricultural Sector in Central and Eastern Europe. Agroinform Kiadó; Budapest, Hungary: 2014. Economic impacts, control and eradication of Bovine Viral Diarrhoea virus; pp. 247–258. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

