Impact of B18R-Encoding Messenger Ribonucleic Acid Co-Delivery on Neutralizing Antibody Production in Self-Amplifying Messenger Ribonucleic Acid Vaccines
- PMID: 40432146
- PMCID: PMC12115987
- DOI: 10.3390/vaccines13050537
Impact of B18R-Encoding Messenger Ribonucleic Acid Co-Delivery on Neutralizing Antibody Production in Self-Amplifying Messenger Ribonucleic Acid Vaccines
Abstract
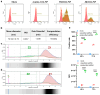
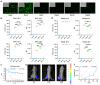
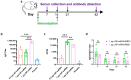
Objectives: The COVID-19 pandemic has brought mRNA vaccines to the forefront due to their widespread use. In this study, we explored the potential advantages of the self-amplifying mRNA (saRNA) vaccine over conventional mRNA vaccines. Methods: Initially, we optimized lipid nanoparticle formulations and employed dT20 affinity chromatography purification to improve the intracellular expression of saRNA. Subsequently, we demonstrated that saRNA exhibited sustained expression for up to one month, both in vitro and in vivo, in contrast to mRNA. Finally, we developed a saRNA-based COVID-19 vaccine and achieved superior immune protection in mice compared to mRNA vaccine by co-delivering the B18R-encoding mRNA. Results: The co-delivery of B18R-mRNA with the saRNA vaccine significantly enhanced neutralizing antibody responses, outperforming those induced by the mRNA vaccine alone. This co-delivery strategy effectively regulated the early innate immune activation triggered by saRNA, facilitating a more robust adaptive immune response. Conclusions: The optimization strategies we used in this study highlight the potential of saRNA vaccines to offer stronger and more durable immune protection. The insights gained from this study not only promote the advancement of saRNA vaccine development but also provide practical guidance for their broader application in the fight against infectious diseases.
Keywords: B18R; chromatography; lipid nanoparticles; self-amplifying mRNA; vaccine.
Conflict of interest statement
Two authors are affiliated with companies but have no potential interest relationship: “Author Min Liang was employed by the company Beijing Syngenbio, Co., Ltd., Beijing, China. Author Gan Liu was employed by the company Beijing Syngentech Co., Ltd., Beijing, China. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest”.
Figures

References
-
- Perri S., Greer C.E., Thudium K., Doe B., Legg H., Liu H., Romero R.E., Tang Z., Bin Q., Dubensky T.W., et al. An alphavirus replicon particle chimera derived from venezuelan equine encephalitis and sindbis viruses is a potent gene-based vaccine delivery vector. J. Virol. 2003;77:10394–10403. doi: 10.1128/JVI.77.19.10394-10403.2003. - DOI - PMC - PubMed
-
- Fleeton M.N., Chen M., Berglund P., Rhodes G., Parker S.E., Murphy M., Atkins G.J., Liljeström P. Self-replicative RNA vaccines elicit protection against influenza A virus, respiratory syncytial virus, and a tickborne encephalitis virus. J. Infect. Dis. 2001;183:1395–1398. doi: 10.1086/319857. - DOI - PubMed
LinkOut - more resources
Full Text Sources

