A Modified Variant of Fasciola hepatica FhSAP-2 (mFhSAP-2) as a Recombinant Vaccine Candidate Induces High-Avidity IgG2c Antibodies and Enhances T Cell Activation in C57BL/6 Mice
- PMID: 40432154
- PMCID: PMC12115747
- DOI: 10.3390/vaccines13050545
A Modified Variant of Fasciola hepatica FhSAP-2 (mFhSAP-2) as a Recombinant Vaccine Candidate Induces High-Avidity IgG2c Antibodies and Enhances T Cell Activation in C57BL/6 Mice
Abstract
Background/objectives: In the past, FhSAP-2, an 11.5 kDa recombinant protein belonging to the Fasciola hepatica saposin-like/NK-lysin family, has been shown to induce over 60% partial protection in immunized rabbits and mice when challenged with F. hepatica metacercariae. However, despite FhSAP-2 being a promising vaccine candidate, its hydrophobic nature has made its purification a challenging process. The present study aimed to determine whether a modified 9.8 kDa variant of protein (mFhSAP-2), lacking a string of 16 hydrophobic amino acids at the amino terminus and a dominant Th1 epitope, could retain its immunogenic and Th1-inducing properties.
Methods: RAW264.7 cells were stimulated with mFhSAP-2, and TNFα levels were determined. C57BL/6 mice were immunized with mFhSAP-2 alone or emulsified with Montanide ISA50. Total anti-mFhSAP-2 IgG subtypes, along with their avidity and titers, were measured using ELISA. The T cell proliferation index and levels of CD4+/CD8+ and IFNγ/IL-4 ratios were determined.
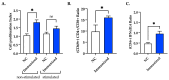
Results: In vitro, mFhSAP-2 induced dose-dependent TNFα production in RAW264.7 cells. In vivo, mice immunized with mFhSAP-2 or mFhSAP-2+ISA50 developed high-avidity IgG2a and IgG2c antibodies at levels that were significantly higher than IgG1 antibody levels. However, the mFhSAP-2+ISA50 formulation induced higher and more homogenous antibody titers than mFhSAP-2, suggesting that an adjuvant may be required to enhance mFhSAP-2 immunogenicity. Immunization with mFhSAP-2+ISA50 also induced significantly higher activated CD4+/CD8+ T cell ratios and IFNγ/IL-4 ratios compared to naïve mice.
Conclusions: Our results demonstrate that mFhSAP-2 retained its immunogenicity and Th1-polarizing properties, which were enhanced by the Montanide ISA50 adjuvant. The present study highlights the feasibility of inducing Th1-associated immune responses in mice using mFhSAP-2 as an antigen. Further studies are required to assess the potential application of the mFhSAP-2+ISA50 formulation as a vaccine against F. hepatica in natural hosts such as cattle and sheep, which could contribute to improved control and aid in the prevention and eradication of F. hepatica infection.
Keywords: C57BL6; FhSAP-2; IFNγ; IgG2a; IgG2c; avidity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Fasciola hepatica: humoral and cytokine responses to a member of the saposin-like protein family following delivery as a DNA vaccine in mice.Exp Parasitol. 2005 Aug;110(4):374-83. doi: 10.1016/j.exppara.2005.03.029. Exp Parasitol. 2005. PMID: 15907838
-
Adjuvant-enhanced antibody and cellular responses to inclusion bodies expressing FhSAP2 correlates with protection of mice to Fasciola hepatica.Exp Parasitol. 2016 Jan;160:31-8. doi: 10.1016/j.exppara.2015.11.002. Epub 2015 Nov 26. Exp Parasitol. 2016. PMID: 26632503 Free PMC article.
-
Fasciola hepatica: identification of CD4+ T-helper epitopes from the 11.5 kDa saposin-like protein SAP-2 using synthetic peptides.Exp Parasitol. 2007 Sep;117(1):65-73. doi: 10.1016/j.exppara.2007.03.012. Epub 2007 Mar 27. Exp Parasitol. 2007. PMID: 17475253 Free PMC article.
-
Measuring of IgG2c isotype instead of IgG2a in immunized C57BL/6 mice with Plasmodium vivax TRAP as a subunit vaccine candidate in order to correct interpretation of Th1 versus Th2 immune response.Exp Parasitol. 2020 Sep;216:107944. doi: 10.1016/j.exppara.2020.107944. Epub 2020 Jun 30. Exp Parasitol. 2020. PMID: 32619431
-
Naltrexone; as an efficient adjuvant in induction of Th1 immunity and protection against Fasciola hepatica infection.Exp Parasitol. 2018 Jun;189:66-71. doi: 10.1016/j.exppara.2018.04.015. Epub 2018 Apr 27. Exp Parasitol. 2018. PMID: 29729492
References
-
- Vazquez A.A., de Vargas M., Alba A., Sanchez J., Alda P., Sabourin E., Vittecoq M. Reviewing Fasciola hepatica transmission in the West Indies and novel perceptions from experimental infections of sympatric vs. allopatric snail/fluke combinations. Vet. Parasitol. 2019;275:108955. doi: 10.1016/j.vetpar.2019.108955. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

