Microtubule polymerization generates microtentacles important in circulating tumor cell invasion
- PMID: 40432209
- PMCID: PMC12256914
- DOI: 10.1016/j.bpj.2025.05.018
Microtubule polymerization generates microtentacles important in circulating tumor cell invasion
Abstract
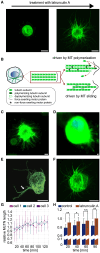
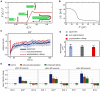
Circulating tumor cells (CTCs) have crucial roles in the spread of tumors during metastasis. A decisive step is the extravasation of CTCs from the blood stream or lymph system, which depends on the ability of cells to attach to vessel walls. Recent work suggests that such adhesion is facilitated by microtubule (MT)-based membrane protrusions called microtentacles (McTNs). However, how McTNs facilitate such adhesion and how MTs can generate protrusions in CTCs remain unclear. By combining fluorescence recovery after photobleaching experiments and simulations we show that polymerization of MTs provides the main driving force for McTN formation, whereas the contribution of MTs sliding with respect to each other is minimal. Further, the forces exerted on the McTN tip result in curvature, as the MTs are anchored at the other end in the MT organizing center. When approaching vessel walls, McTN curvature is additionally influenced by the adhesion strength between the McTN and wall. Moreover, increasing McTN length, reducing its bending rigidity, or strengthening adhesion enhances the cell-wall contact area and, thus, promotes cell attachment to vessel walls. Our results demonstrate a link between the formation and function of McTNs, which may provide new insight into metastatic cancer diagnosis and therapy.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450
-
Ventilator Management(Archived).2023 Mar 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2023 Mar 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 28846232 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Maternal and neonatal outcomes of elective induction of labor.Evid Rep Technol Assess (Full Rep). 2009 Mar;(176):1-257. Evid Rep Technol Assess (Full Rep). 2009. PMID: 19408970 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources

