Assessment of biophysical properties of the first-in-class anti-cancer IgE antibody drug MOv18 IgE demonstrates monomeric purity and stability
- PMID: 40432600
- PMCID: PMC12123954
- DOI: 10.1080/19420862.2025.2512211
Assessment of biophysical properties of the first-in-class anti-cancer IgE antibody drug MOv18 IgE demonstrates monomeric purity and stability
Abstract
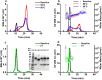
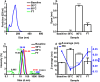
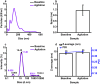
Therapeutic monoclonal antibodies, which are almost exclusively IgG isotypes, show significant promise but are prone to poor solution stability, including aggregation and elevated solution viscosity at dose-relevant concentrations. Recombinant IgE antibodies are emerging cancer immunotherapies. The first-in-class MOv18 IgE, recognizing the cancer-associated antigen folate receptor-alpha (FRα), completed a Phase 1 clinical trial in patients with solid tumors, showing early signs of efficacy at a low dose. The inaugural process development and scaled manufacture of MOv18 IgE for clinical testing were undertaken with little baseline knowledge about the solution phase behavior of recombinant IgE at dose-relevant concentrations. We evaluated MOv18 IgE physical stability in response to environmental and formulation stresses encountered throughout shelf life. We analyzed changes in physical stability using multiple orthogonal analytical techniques, including particle tracking analysis, size exclusion chromatography, and multidetector flow field flow fractionation hyphenated with UV. We used dynamic and multiangle light scattering to profile aggregation status. Formulation at pH 6.5, selected for use in the Phase 1 trial, resulted in high monomeric purity and no submicron proteinaceous particulates. Formulation at pH 5.5 and 7.5 induced significant submicron and sub-visible particle formation. IgE formulation was resistant to aggregation in response to freeze-thaw stress, retaining high monomeric purity. Exposure to thermal stress at elevated temperatures resulted in loss of monomeric purity and aggregation. Agitation stress-induced submicron and subvisible aggregation, but monomeric purity was not significantly affected. MOv18 IgE retains monomeric purity in response to formulation and stress conditions, confirming stability. Our results offer crucial guidance for future IgE-based drug development.
Keywords: Cancer immunotherapy; IgE; formulation; immunoglobulin; monoclonal antibody; particle size; protein aggregation; stability.
Conflict of interest statement
J.S. and S.N.K. are founders and shareholders of Epsilogen Ltd. H.J.B. is employed through a fund provided by Epsilogen Ltd. J.C. has been employed through a fund provided by Epsilogen Ltd. All other authors declare no conflict of interest.
Figures





Similar articles
-
Physiochemical and functional evaluation of the first-in-class anti-cancer IgE antibody drug, MOv18, through process development and good manufacturing practice production.MAbs. 2025 Dec;17(1):2451295. doi: 10.1080/19420862.2025.2451295. Epub 2025 Jan 20. MAbs. 2025. PMID: 39833133
-
Harnessing engineered antibodies of the IgE class to combat malignancy: initial assessment of FcɛRI-mediated basophil activation by a tumour-specific IgE antibody to evaluate the risk of type I hypersensitivity.Clin Exp Allergy. 2011 Oct;41(10):1400-13. doi: 10.1111/j.1365-2222.2011.03770.x. Epub 2011 May 16. Clin Exp Allergy. 2011. PMID: 21569129
-
An immunologically relevant rodent model demonstrates safety of therapy using a tumour-specific IgE.Allergy. 2018 Dec;73(12):2328-2341. doi: 10.1111/all.13455. Epub 2018 Oct 8. Allergy. 2018. PMID: 29654623 Free PMC article.
-
Recombinant IgE antibodies for passive immunotherapy of solid tumours: from concept towards clinical application.Cancer Immunol Immunother. 2012 Sep;61(9):1547-64. doi: 10.1007/s00262-011-1162-8. Epub 2011 Dec 3. Cancer Immunol Immunother. 2012. PMID: 22139135 Free PMC article. Review.
-
Therapeutic IgE Antibodies: Harnessing a Macrophage-Mediated Immune Surveillance Mechanism against Cancer.Cancer Res. 2017 Jun 1;77(11):2779-2783. doi: 10.1158/0008-5472.CAN-17-0428. Epub 2017 May 19. Cancer Res. 2017. PMID: 28526770 Review.
References
-
- Markov SD, Caffrey TC, O’Connell KA, Grunkemeyer JA, Shin S, Hanson R, Patil PP, Shukla SK, Gonzalez D, Crawford AJ, et al. IgE-based therapeutic combination enhances antitumor response in preclinical models of pancreatic cancer. Mol Cancer Ther. 2021;20(12):2457–2468. doi: 10.1158/1535-7163.MCT-21-0368. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
