Deleterious effect of Pseudomonas aeruginosa on F508del-CFTR rescued by elexacaftor/tezacaftor/ivacaftor is clinical strain-dependent in patient-derived nasal cells
- PMID: 40432819
- PMCID: PMC12107384
- DOI: 10.1183/23120541.00970-2024
Deleterious effect of Pseudomonas aeruginosa on F508del-CFTR rescued by elexacaftor/tezacaftor/ivacaftor is clinical strain-dependent in patient-derived nasal cells
Abstract
Background: The triple cystic fibrosis transmembrane conductance regulator (CFTR) modulators combination elexacaftor/tezacaftor/ivacaftor (ETI) has been approved for people with cystic fibrosis (pwCF) bearing at least one F508del allele. Despite the development of CFTR modulators having dramatically improved respiratory outcomes in pwCF, clinical studies have showed variable responses to this drug formulation. Of note, airway inflammation and bacterial colonisation persist in the upper and lower respiratory tract even in ETI-treated patients.
Methods: We first tested the clinical exoproducts (EXO) of Pseudomonas aeruginosa isolated from 15 CF patients in wild-type (WT) and F508del-CFTR CF bronchial epithelial (CFBE) cells. We were then prompted to evaluate the effects of EXO in ex-vivo patient-derived tissues. Therefore, we cultured primary nasal epithelial cells (HNECs) with EXO isolated from the corresponding pwCF to mimic the native status of CF airway.
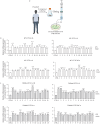
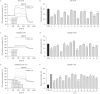
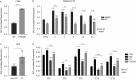
Results: We found that EXO variably decreased WT-, F508del- and ETI-dependent F508del-CFTR function and increased proinflammatory cytokines and reactive oxygen species (ROS) levels in a clinical strain-specific manner. Similarly, we observed a variable reduction of F508del-CFTR function in presence or absence of ETI and upregulation of proinflammatory cytokines and ROS levels. Interestingly, HNECs treated with EXO isolated from the corresponding donor and three different pwCF showed a variable reduction of ETI-dependent F508del-CFTR function mainly due to clinical strains with limited effect of patient background. Furthermore, we demonstrated that ETI pretreatment decreased the cytokines and ROS levels down to the levels of uninfected cells.
Conclusion: These preclinical studies suggest that in vitro screening of patient-specific response to CFTR modulators under infection/inflammation conditions could prove to be a valuable tool to enhance the prediction of clinical response.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Corrector therapies (with or without potentiators) for people with cystic fibrosis with class II CFTR gene variants (most commonly F508del).Cochrane Database Syst Rev. 2020 Dec 17;12(12):CD010966. doi: 10.1002/14651858.CD010966.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2023 Nov 20;11:CD010966. doi: 10.1002/14651858.CD010966.pub4. PMID: 33331662 Free PMC article. Updated.
-
Response to Elexacaftor/Tezacaftor/Ivacaftor in people with cystic fibrosis with the N1303K mutation: Case report and review of the literature.Heliyon. 2024 Feb 28;10(5):e26955. doi: 10.1016/j.heliyon.2024.e26955. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38463894 Free PMC article.
-
The F508del-CFTR trafficking correctors elexacaftor and tezacaftor are CFTR-independent Ca2+-mobilizing agonists normalizing abnormal Ca2+ levels in human airway epithelial cells.Respir Res. 2024 Dec 19;25(1):436. doi: 10.1186/s12931-024-03059-8. Respir Res. 2024. PMID: 39702307 Free PMC article.
-
The rescue of F508del-CFTR by elexacaftor/tezacaftor/ivacaftor (Trikafta) in human airway epithelial cells is underestimated due to the presence of ivacaftor.Eur Respir J. 2022 Feb 24;59(2):2100671. doi: 10.1183/13993003.00671-2021. Print 2022 Feb. Eur Respir J. 2022. PMID: 34266939
-
The clinical effectiveness of elexacaftor/tezacaftor/ivacaftor (ETI) for people with CF without a F508del variant: A systematic review and meta-analysis.J Cyst Fibros. 2024 Sep;23(5):950-958. doi: 10.1016/j.jcf.2024.07.012. Epub 2024 Jul 23. J Cyst Fibros. 2024. PMID: 39048464
References
-
- Lund-Palau H, Turnbull AR, Bush A, et al. . Pseudomonas aeruginosa infection in cystic fibrosis: pathophysiological mechanisms and therapeutic approaches. Expert Rev Respir Med 2016; 10: 685–697. - PubMed
LinkOut - more resources
Full Text Sources
