Renal tubular epithelial cell related partial epithelial-mesenchymal transition in AAⅠ induced renal fibrosis via Wnt7b/β-catenin signaling
- PMID: 40432885
- PMCID: PMC12106489
- DOI: 10.3389/fphar.2025.1571960
Renal tubular epithelial cell related partial epithelial-mesenchymal transition in AAⅠ induced renal fibrosis via Wnt7b/β-catenin signaling
Abstract
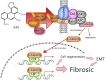
Introduction: This study investigates the pathological progressions in kidneys affected by aristolochic acid nephropathy (AAN) and explores the molecular mechanisms underlying the fibrotic process, specifically focusing on the Wnt7b/β-catenin signaling pathway.
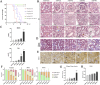
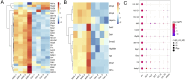
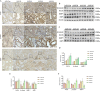
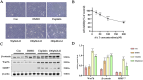
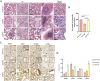
Methods: Both mice and human kidney-2 (HK-2) cells were treated with aristolochic acid I (AAI). In mice, we monitored blood urea nitrogen (BUN), serum creatinine (Scr), kidney injury molecule-1 (KIM-1), pathological modifications of renal tubular epithelial cells (RTECs), and fibrosis degrees during acute/chronic disease phases. Wnt7b/β-catenin expression was evaluated through transcriptome analysis and laboratory assays (immunohistochemistry, Western blotting, immunoelectron microscopy) in acute AAN and cultured cells. Concurrent assays measured representative proteins: Aquaporin 1 (AQP1), Topoisomerase IIα (TOP2A), Vascular Cell Adhesion Molecule-1 (VCAM-1), and α-smooth muscle actin (α-SMA) in chronic AAN RTECs.
Results: AAI increased Scr, BUN, and KIM-1 levels by causing RTEC necrotic shedding in acute stages and promoted renal interstitial fibrosis chronically. Elevated Wnt7b pathway proteins enhanced damaged RTEC regeneration, with regenerated cells expressing mesenchymal proteins VCAM-1 and α-SMA.
Discussion: The Wnt7b/β-catenin signaling pathway connects acute tubule damage to fibrosis, explaining AAN's pathological continuum. These findings clarify how acute injury progresses to chronic fibrosis in AAN.
Keywords: Wnt7b/β-catenin; aristolochic acid nephropathy; damage and repair; epithelial-mesenchymal transition; renal tubular epithelial cell.
Copyright © 2025 Wang, Zheng, Zhang, Li, Xia, Tang, Huang, Li and Lou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Aristolochic acid I abnormally activates the wnt7b/β-catenin signaling pathway and affects the repair of renal tubules.Chem Biol Interact. 2025 Feb 25;408:111413. doi: 10.1016/j.cbi.2025.111413. Epub 2025 Feb 5. Chem Biol Interact. 2025. PMID: 39921188
-
The potential role of aquaporin 1 on aristolochic acid I induced epithelial mesenchymal transition on HK-2 cells.J Cell Physiol. 2018 Jun;233(6):4919-4925. doi: 10.1002/jcp.26310. Epub 2018 Jan 2. J Cell Physiol. 2018. PMID: 29215709
-
PSTPIP2 ameliorates aristolochic acid nephropathy by suppressing interleukin-19-mediated neutrophil extracellular trap formation.Elife. 2024 Feb 5;13:e89740. doi: 10.7554/eLife.89740. Elife. 2024. PMID: 38314821 Free PMC article.
-
Renal Protective Effects of 17β-Estradiol on Mice with Acute Aristolochic Acid Nephropathy.Molecules. 2016 Oct 18;21(10):1391. doi: 10.3390/molecules21101391. Molecules. 2016. PMID: 27763560 Free PMC article.
-
TGF-beta 1/Smads signaling stimulates renal interstitial fibrosis in experimental AAN.J Recept Signal Transduct Res. 2009;29(5):280-5. doi: 10.1080/10799890903078465. J Recept Signal Transduct Res. 2009. PMID: 19640259
References
-
- Cuesta C., Fuentes-Calvo I., Sancho-Martinez S. M., Valentijn F. A., Duwel A., Hidalgo-Thomas O. A., et al. (2022). Urinary KIM-1 correlates with the subclinical sequelae of tubular damage persisting after the apparent functional recovery from intrinsic acute kidney injury. Biomedicines 10 (5), 1106. 10.3390/biomedicines10051106 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

