Primary hyperoxaluria type I diagnosed after a kidney transplant presenting with subcutaneous calcification: a case report of sodium thiosulfate treatment
- PMID: 40432890
- PMCID: PMC12106461
- DOI: 10.3389/fphar.2025.1485024
Primary hyperoxaluria type I diagnosed after a kidney transplant presenting with subcutaneous calcification: a case report of sodium thiosulfate treatment
Abstract
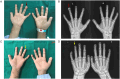
Primary hyperoxaluria (PH) is a rare autosomal recessive disorder that results from the overproduction of endogenous oxalate. The diagnosis of PH is often delayed or missed owing to its rarity, variable clinical expression and other diagnostic challenges. In this study, we report a patient with a frameshift variant, c.823_824dup, in the alanine-glyoxylate aminotransferase (AGXT) gene of PH1 who presented with renal failure recurrence after kidney transplantation, arteriovenous fistula (AVF) occlusion and subcutaneous calcification in adulthood. Skin biopsy revealed heavy deposition of calcium oxalate crystals in subcutaneous tissue without vascular oxalosis. After 6 courses of sodium thiosulfate (STS) treatment, X-rays of the bilateral hands showed the disappearance of subcutaneous calcification on the extremity of the left-hand ring-finger. This case highlights the importance of broad diagnostic testing prior to transplantation in patients who present with end-stage renal disease with unclear etiology. In addition, STS may be useful for PH1 patients with subcutaneous calcium deposits.
Keywords: AGXT gene; primary hyperoxaluria; skin biopsy; sodium thiosulfate; subcutaneous calcification.
Copyright © 2025 Wu, Lu, Wang, Li, Wei, Gong and Tang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Generation and characterization of a novel rat model of primary hyperoxaluria type 1 with a nonsense mutation in alanine-glyoxylate aminotransferase gene.Am J Physiol Renal Physiol. 2021 Mar 1;320(3):F475-F484. doi: 10.1152/ajprenal.00514.2020. Epub 2021 Jan 25. Am J Physiol Renal Physiol. 2021. PMID: 33491567
-
Primary Hyperoxaluria Type 1 (PH1) Presenting With End-Stage Kidney Disease and Cutaneous Manifestations in Adulthood: A Case Report.Can J Kidney Health Dis. 2021 Nov 22;8:20543581211058931. doi: 10.1177/20543581211058931. eCollection 2021. Can J Kidney Health Dis. 2021. PMID: 34840803 Free PMC article.
-
Identification of a novel AGXT gene mutation in primary hyperoxaluria after kidney transplantation failure.Transpl Immunol. 2016 Nov;39:60-65. doi: 10.1016/j.trim.2016.08.008. Epub 2016 Aug 25. Transpl Immunol. 2016. PMID: 27568336
-
Infant primary hyperoxaluria type 1: A case report and literature review.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024 Jun 28;49(6):856-862. doi: 10.11817/j.issn.1672-7347.2024.230582. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 39311781 Free PMC article. Review. Chinese, English.
-
ENDOCRINE MANIFESTATIONS OF PRIMARY HYPEROXALURIA.Endocr Pract. 2017 Dec;23(12):1414-1424. doi: 10.4158/EP-2017-0029. Epub 2017 Nov 16. Endocr Pract. 2017. PMID: 29144803 Review.
References
Publication types
LinkOut - more resources
Full Text Sources

