FDA Device Approval-What You Were Not Taught in Training
- PMID: 40432952
- PMCID: PMC12088133
- DOI: 10.55275/JPOSNA-2023-771
FDA Device Approval-What You Were Not Taught in Training
Abstract
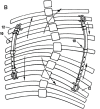
Medical devices are ubiquitous in the practice of pediatric orthopaedic surgery, but few surgeons receive any formal training or education on the process of bringing a medical device to market for pediatric orthopaedic patients. Innovation in the field of medical devices has led to significant improvement in the care of pediatric orthopaedic patients. The American Academy of Orthopaedic Surgeons (AAOS) and the Pediatric Orthopaedic Society of North America (POSNA) recognize the importance of making innovative medical devices available to pediatric patients and have made advocacy an important aspect of their mission and relationship with industry and governmental regulatory organizations. Understanding the history, structure, and pathways to market approval are critical to encouraging the innovation of novel devices and techniques and improving the care of pediatric orthopaedic patients. Orthopaedists should have a command of the concepts of adverse event reporting as well as an understanding of the on- and off-label uses of medical devices as this is helpful for discussion of care and informed consent for the use of such devices. Due to the relative rarity of the diseases addressed, pediatric orthopaedists should understand the framework of the humanitarian device exemption as well. These concepts can be synergized into some successful improvements in care for pediatric orthopaedic patients. One such success story in recent history was the introduction of the Vertical Expandable Titanium Prosthetic Rib (VEPTR) system by Dr. Robert Campell Jr. Key Concepts•The Food and Drug Administration (FDA) is the federal regulatory agency responsible for the approval and regulation of medical devices in the United States.•The FDA classifies the risk of medical devices as low, medium, and high, and pathways to approval for devices vary based on this risk classification.•One common exemption of importance to pediatric orthopaedic surgeons is the humanitarian device exemption, which can aid in the development and use of devices for the often relatively rare conditions faced by pediatric orthopaedic patients.
© 2023 JPOSNA. Published by Elsevier on behalf of the Pediatric Orthopaedic Society of North America.
Figures
References
-
- Federal Food, Drug, and Cosmetic Act (FD&C Act). United States Code (U.S.C.) Title 21, Chapter 9.
-
- Sastry A. Overview of the US FDA medical device approval process. Curr Cardiol Rep. 2014;16:1–5. - PubMed
-
- Darrow J.J., Avorn J., Kesselheim A.S. FDA regulation and approval of medical devices: 1976-2020. JAMA. 2021;326(5):420–432. - PubMed
-
- Buch B. FDA medical device approval: things you didn't learn in medical school or residency. Am J Orthop (Belle Mead NJ). 2007;36(8):407–412. - PubMed
Publication types
LinkOut - more resources
Full Text Sources