Microecological mechanisms of mountainous forest cultivated ginseng growth vigor and saponin accumulation, and the characterization of bionic microbial fertilizer
- PMID: 40432972
- PMCID: PMC12106421
- DOI: 10.3389/fmicb.2025.1548481
Microecological mechanisms of mountainous forest cultivated ginseng growth vigor and saponin accumulation, and the characterization of bionic microbial fertilizer
Abstract
Introduction: A study on the soil microecological mechanisms influencing the growth vigor and saponin accumulation of mountainous forest cultivated ginseng (MFCG) under various forest types.
Methods: Using MFCG from different forest types as experimental material, the correlation and functional analysis of MFCG growth vigor, ginseng saponin content, and soil nutrient elements in their rhizosphere were conducted to clarify the soil microecological mechanisms by which different forest types affect the growth vigor and saponin accumulation of understory ginseng. Based on these microecological mechanisms, a bionic microbial fertilizer was developed and characterized.
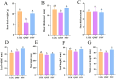
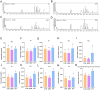
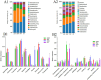
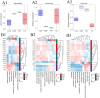
Results: The agronomic traits and saponin content (Re, Rc, Rb2, and Rb3) of MFCG in the Pinus sylvestris var. mongholica Litv. (PSV) group were significantly higher than those in the Quercus mongolica Fisch. ex Ledeb. (QMF) and Larix gmelinii (Rupr.) Kuzen (LGK) groups (p < 0.05). The total content of these four monomeric saponins in the PSV group was 35.1 and 45.56% higher than that in the QMF and LGK groups, respectively. Significant differences (p < 0.05) were observed between the PSV group and the QMF and LGK groups in terms of the rhizosphere soil microbial diversity and physicochemical indicators such as nutrient elements. The agronomic traits and saponin content of MFCG were positively correlated with chemical indicators in the rhizosphere soil, including Cu, Ca, Mg, Zn, B, Fe, Mo, Mn, Organic matter (OM), Available phosphorus (AP), Available nitrogen (AN), and Available potassium (AK). Based on the microbial diversity and nutrient elements positively correlated with MFCG in the rhizosphere soil, a bionic microbial fertilizer formula was optimized.
Discussion: The microecological mechanism behind the growth vigor and saponin accumulation of understory ginseng involves an increase in beneficial microorganisms and nutrient elements, along with a reduction in harmful microorganisms and detrimental elements. The bionic microbial fertilizer promoted MFCG growth and saponin accumulation while improving soil nutrient levels, bulk density, and water-holding capacity.
Keywords: bionic microbial fertilizer; ginseng; growth vigor; microecological mechanisms; saponin accumulation.
Copyright © 2025 Pang, Gao, Zhuang, Li, Zhao and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Ali Q., Ali M., Jing H., Hussain A., Manghwar H., Ali M., et al. (2024). Power of plant microbiome: a sustainable approach for agricultural resilience. Plant Stress 14:100681. doi: 10.1016/j.stress.2024.100681 - DOI
LinkOut - more resources
Full Text Sources

