Synthesis of an amphiphilic bi-prodrug based on gossypol and cytarabine for drug self-delivery
- PMID: 40433031
- PMCID: PMC12108794
- DOI: 10.1039/d5ra02555a
Synthesis of an amphiphilic bi-prodrug based on gossypol and cytarabine for drug self-delivery
Abstract
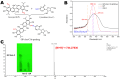
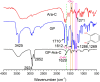
Drug-drug conjugates have become a research hotspot in nanomedicine. An amphiphilic assembled drug-drug conjugate can achieve drug self-delivery without any carriers and change the distribution of free drugs. Herein, we synthesized a pH-responsive bi-prodrug of gossypol (GP) and cytarabine (Ara-C) for cancer therapy. FT-IR, UV-vis, TLC and MS confirmed that GP-Ara-C was successfully synthesized. The nanoparticle size of GP-Ara-C is approximately 100 nm, and the stability of GP-Ara-C is maintained for 6 days in pH 7.4 phosphate-buffered saline (PBS). HeLa cell viability results showed that GP-Ara-C exhibited anticancer ability similar to that of free GP. Cellular uptake demonstrated that GP-Ara-C could be distributed in cell nuclei at 7 h. Altogether, this amphiphilic GP-Ara-C could be assembled into nanomedicine in an aqueous environment and could be a candidate for cancer therapy in clinics.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Li S. Jiang M. Wang L. Yu S. Biomed. Pharmacother. 2020;129:110389. - PubMed
-
- Zhang Z. Yue Y. Xu L. Wang Y. Geng W. Li J. Kong X. Zhao X. Zheng Y. Zhao Y. Shi L. Guo D. Liu Y. Adv. Mater. 2021;33:2007719. - PubMed
-
- Hu M. Huang P. Wang Y. Su Y. Zhou L. Zhu X. Yan D. Bioconjugate Chem. 2015;26(12):2497–2506. - PubMed
LinkOut - more resources
Full Text Sources

