Changes in female cancer diagnostic billing rates over the COVID-19 period in the Ontario Health Insurance Plan
- PMID: 40433105
- PMCID: PMC12106997
- DOI: 10.1177/17588359251339919
Changes in female cancer diagnostic billing rates over the COVID-19 period in the Ontario Health Insurance Plan
Abstract
Background: The initial response to coronavirus disease 2019 (COVID-19) in Ontario included suspension of cancer screening programs and deferral of diagnostic procedures and many treatments. Although the short-term impact of these measures on female cancers is well documented, few studies have assessed the mid- to long-term impacts.
Objectives: To compare annual billing prevalence and incidence rates of female cancers during the COVID-19 period (2020-2022) to pre-COVID-19 levels (2015-2019).
Design: Retrospective analysis of aggregated claims data for female cancer diagnostic codes from the Ontario Health Insurance Plan (OHIP).
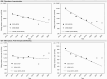
Methods: Linear regression analysis was used to fit pre-COVID-19 (2015-2019) data for each OHIP billing code and extrapolate counterfactual values for the years of 2020-2022. Excess billing rates were calculated as the difference between projected and actual rates for each year.
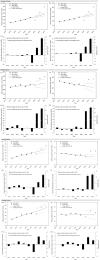
Results: In 2020, OHIP billing prevalence rates for cervical, breast, uterine, and ovarian cancers decreased relative to projected values for that year by -50.7/100k, -13.9/100k, -3.5/100k, and -3.8/100k, respectively. The reverse was observed in 2021 with rate increases of 47.8/100k, 59.1/100k, 2.5/100k, and 3.7/100k, respectively. In 2022, the excesses were further amplified, especially for cervical and breast cancers (111.2/100k and 78.67/100k, respectively). The net excess patient billing rate for 2020-2022 was largely positive for all female cancer types (108.3/100k, 123.7/100k, 5.2/100k, and 1.8/100k, respectively). Analysis of billing incidence rates showed similar trends.
Conclusion: The expected female cancer billing rate decreases in 2020 were followed by large increases in 2021 and 2022, resulting in a cumulative excess during the COVID-19 period. Further research is required to assess the nature of these changes.
Keywords: COVID-19; breast cancer; diagnostic claims; gynecological cancers; public health measures.
© The Author(s), 2025.
Conflict of interest statement
A.D.A. has been employed and/or served in a leadership position for Doctors MB, GOC, and CCMB PT committee, has served in a consultancy or advisory role for AstraZeneca, Eisai, Abbvie, Merck, and GSK, and has received research funding from Merck, Pfizer, AstraZeneca, Clovis, and CCMB Foundation. C.B.-M. has received honoraria from and served in a consultancy or advisory role for Astellas, Amgen, AstraZeneca, Beigene, Gilead Sciences, Pfizer, Novartis, Eli Lilly, Merck, BMS, Sanofi, and Knight Therapeutics, and has received research funding from Pfizer, AstraZeneca, Gilead Sciences, Eli Lilly, and Novartis. N.L. has served in a leadership position for Provincial Breast Systemic, has received research funding, and has served in a consultancy or advisory role for AstraZeneca, Eli Lilly, Gilead Sciences, Knight Therapeutics, Merck, Novartis, and Pfizer, and has received honoraria from AstraZeneca, Eli Lilly, Gilead Sciences, Knight Therapeutics, Merck, Novartis, and Pfizer. I.M. has no conflicts of interest to disclose. D.M. has no conflicts of interest to disclose. A.S. has been employed and/or served in a leadership position for the Society of Canadian Colposcopists, International Society for the Study of Vulvovaginal Disease—North American Branch, and the International Federation of Colposcopy and Cervical Pathology, and has served in a consultancy or advisory role for Procter and Gamble. A.V.T. has received honoraria from and has served in a consultancy or advisory role for AstraZeneca, Merck, GSK, Eisai, and Abbvie, and has received research funding from AstraZeneca.
Figures
References
-
- World Health Organization. WHO Director-General’s statement on IHR Emergency Committee on Novel Coronavirus (2019-nCoV), https://www.who.int/director-general/speeches/detail/who-director-genera...) (2020, accessed 17 May 2024).
-
- Canadian Institute for Health Information. Canadian COVID-19 intervention timeline, https://www.cihi.ca/en/canadian-covid-19-intervention-timeline (2022, accessed 17 May 2024).
-
- Mathieu E, Ritchie H, Rodés-Guirao L, et al.. COVID-19: stringency index, https://ourworldindata.org/covid-stringency-index (2023, accessed 5 August 2024).
-
- Government of Ontario. Ontario data catalogue—COVID-19 vaccine data in Ontario, https://data.ontario.ca/dataset/covid-19-vaccine-data-in-ontario (2023, accessed 1 May 2023).
LinkOut - more resources
Full Text Sources

