Plasma α-synuclein domain profiles across α-synucleinopathies
- PMID: 40433114
- PMCID: PMC12107063
- DOI: 10.1093/braincomms/fcaf189
Plasma α-synuclein domain profiles across α-synucleinopathies
Abstract
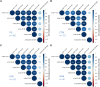
The differential diagnosis of α-synucleinopathies, including Parkinson's disease, dementia with Lewy bodies (DLB) and multiple system atrophy (MSA), remains challenging due to overlapping clinical features and the absence of reliable biomarkers. We developed a targeted mass spectrometry assay to profile α-synuclein peptides in plasma from Parkinson's disease (n = 82), DLB (n = 32), MSA (n = 8) and controls (n = 21). We hypothesized that disease-specific truncations or post-translational modifications would alter levels of non-modified α-synuclein peptides across α-synucleinopathies. The assay quantified non-modified peptides derived from the N-terminus and non-amyloid component (NAC) domain, regions implicated in aggregate formation. Although peptide levels were consistent across disease groups, a distinct NAC domain pattern observed in MSA may reflect unique pathological processes. This study presents the first blood-based profiling of α-synuclein peptides in these disorders, offering a basis for further investigation into disease mechanisms. Refinement of the assay to include post-translational modifications could enhance understanding of α-synucleinopathies and support future biomarker development.
Keywords: Lewy bodies; Parkinson’s disease; mass spectrometry; multiple system atrophy; α-synuclein.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures




References
-
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. - PubMed
-
- Williams-Gray CH, Mason SL, Evans JR, et al. The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatry. 2013;84(11):1258–1264. - PubMed
-
- Tu PH, Galvin JE, Baba M, et al. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble α-synuclein. Ann Neurol. 1998;44(3):415–422. - PubMed
LinkOut - more resources
Full Text Sources
