"Remodeling the intestinal immune microenvironment": immune regulation and tissue regeneration by mesenchymal stem/stromal cells in the repair microenvironment of inflammatory bowel disease
- PMID: 40433382
- PMCID: PMC12106535
- DOI: 10.3389/fimmu.2025.1543702
"Remodeling the intestinal immune microenvironment": immune regulation and tissue regeneration by mesenchymal stem/stromal cells in the repair microenvironment of inflammatory bowel disease
Abstract
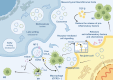
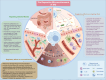
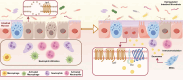
The global prevalence of inflammatory bowel disease (IBD) has significantly increased in recent decades. IBD is a long-term, recurring, gastrointestinal inflammatory condition that mainly comprises two primary clinical types: ulcerative colitis and Crohn's disease. The current treatment paradigm for IBD primarily focuses on symptom management. However, this approach does not support mucosal epithelial repair, maintenance of barrier homeostasis, or regulation of biological functions in the gut. Conventional therapies rely on the frequent use of high-dose medications, including antibiotics, nonsteroidal anti-inflammatory drugs, biological agents, and immunomodulators. Recently, mesenchymal stem/stromal cells (MSCs) have gained interest in tissue regeneration owing to their unique ability to differentiate and secrete regulatory factors, including extracellular vesicles (EVs), which play crucial roles in abnormal organization. Various routes of administration have been explored in preclinical and clinical studies to deliver MSCs from diverse tissue sources. The routes include intraperitoneal, intravenous, and local (intracolonic or rectal) delivery. The MSCs employed were obtained from various tissues, including bone marrow, umbilical cord, and adipose tissue. This article reviews the research framework for the application of MSCs and EVs secretion in the treatment of IBD, emphasizing key immunological effects, such as immune microenvironment regulation, intestinal barrier stabilization, and therapeutic approaches targeting intestinal barrier disorders. The discussion primarily focuses on the advantages of MSCs over other biologics, impairment of gut mucosal tissue-resident mesenchymal stem cells in IBD development, immune targets (at the cellular and molecular levels) within the framework of IBD, and the reparative effects of MSCs in the microenvironment of IBD. We aimed to present an overview of the current trends in MSC research and therapy, as well as to identify the challenges and future directions that must be addressed to advance research on MSC-mediated therapeutic strategies for IBD.
Keywords: biological therapies; inflammatory bowel disease; intestinal immune microenvironment; mesenchymal stem/stromal cells; tissue regeneration.
Copyright © 2025 Li, Zhang, Du, Shen, Liu and Jing.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles: A focus on inflammatory bowel disease.Clin Transl Med. 2024 Nov;14(11):e70075. doi: 10.1002/ctm2.70075. Clin Transl Med. 2024. PMID: 39488745 Free PMC article. Review.
-
A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6.Stem Cell Res Ther. 2021 May 29;12(1):315. doi: 10.1186/s13287-021-02404-8. Stem Cell Res Ther. 2021. PMID: 34051868 Free PMC article.
-
Extracellular vesicles-mediated interaction within intestinal microenvironment in inflammatory bowel disease.J Adv Res. 2021 Jul 8;37:221-233. doi: 10.1016/j.jare.2021.07.002. eCollection 2022 Mar. J Adv Res. 2021. PMID: 35499059 Free PMC article. Review.
-
Mesenchymal Stromal Cells: New Generation Treatment of Inflammatory Bowel Disease.J Inflamm Res. 2024 May 22;17:3307-3334. doi: 10.2147/JIR.S458103. eCollection 2024. J Inflamm Res. 2024. PMID: 38800593 Free PMC article. Review.
-
MIS416 Enhances Therapeutic Functions of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Against Experimental Colitis by Modulating Systemic Immune Milieu.Front Immunol. 2018 May 28;9:1078. doi: 10.3389/fimmu.2018.01078. eCollection 2018. Front Immunol. 2018. PMID: 29892282 Free PMC article.
Cited by
-
Review: progression and heterogeneity of multiple sources of mesenchymal stromal cells for the treatment of rheumatoid arthritis.Stem Cell Res Ther. 2025 Jul 9;16(1):358. doi: 10.1186/s13287-025-04515-y. Stem Cell Res Ther. 2025. PMID: 40629483 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

