The recent advances in vaccine adjuvants
- PMID: 40433383
- PMCID: PMC12106398
- DOI: 10.3389/fimmu.2025.1557415
The recent advances in vaccine adjuvants
Abstract
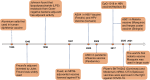
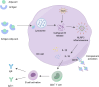
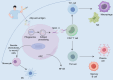
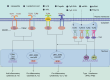
Vaccine adjuvants, as key components in enhancing vaccine immunogenicity, play a vital role in modern vaccinology. This review systematically examines the historical evolution and mechanisms of vaccine adjuvants, with particular emphasis on innovative advancements in aluminum-based adjuvants, emulsion-based adjuvants, and nucleic acid adjuvants (e.g., CpG oligonucleotides). Specifically, aluminum adjuvants enhance immune responses through particle formation/antigen adsorption, inflammatory cascade activation, and T-cell stimulation. Emulsion adjuvants amplify immunogenicity via antigen depot effects and localized inflammation, while nucleic acid adjuvants like CpG oligonucleotides directly activate B cells and dendritic cells to promote Th1-type immune responses and memory T-cell generation. The article further explores the prospective applications of these novel adjuvants in combating emerging pathogens (including influenza and SARS-CoV-2), particularly highlighting their significance in improving vaccine potency and durability. Moreover, this review underscores the critical importance of adjuvant development in next-generation vaccine design and provides theoretical foundations for creating safer, effective adjuvant.
Keywords: adjuvants; combinatorial adjuvant strategies; delivery systems; immunostimulants; vaccine.
Copyright © 2025 Xing, Zhao, Li, Fang, Sun, Zhang and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

