SARM1: a key multifaceted component in immunoregulation, inflammation and neurodegeneration
- PMID: 40433385
- PMCID: PMC12106052
- DOI: 10.3389/fimmu.2025.1521364
SARM1: a key multifaceted component in immunoregulation, inflammation and neurodegeneration
Abstract
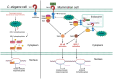
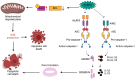
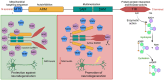
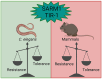
The downstream signaling pathways of TLR activation involve a family of adaptor proteins, including MYD88, TIRAP, TRIF, TRAM, and SARM1. The first four proteins stimulate inflammatory and antiviral responses, playing crucial roles in innate immunity against various pathogens. In contrast, SARM1 promotes immunity to microorganisms in invertebrate animals independently of TLRs, and negatively regulates inflammatory responses in metazoan organisms. SARM1 inhibits TRIF, reduces the activation of various inflammasomes, and induces mitochondrial damage and cell death to eliminate hyperactivated cells. This regulation is essential to ensure timely control of immune responses and to prevent excessive inflammation. Recently, it was discovered that SARM1 can hydrolyze NAD, a critical component of cellular metabolism. The reduction of NAD levels by SARM1 is linked to the progression of Wallerian degeneration following neuronal injury and may also play a role in the immunoregulation of lymphoid and myeloid cells. Since SARM1 can be pharmacologically modulated, it presents promising opportunities for developing treatments for inflammatory and neurodegenerative diseases.
Keywords: cell death; immunometabolism; immunoregulation; innate immunity; neurodegenerative disease.
Copyright © 2025 Oliveira, Honório da Silva, Vieira, Moreira, Bandeira, Ramos, Silva and Câmara.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

