Increased reproductive outcomes after optimized sperm preparation
- PMID: 40433542
- PMCID: PMC12107353
- DOI: 10.3389/fcell.2025.1596421
Increased reproductive outcomes after optimized sperm preparation
Abstract
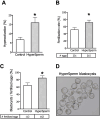
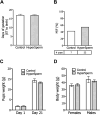
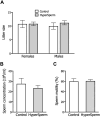
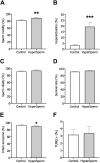
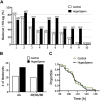
A key factor to the success of in vitro fertilization (IVF) is the preparation of human sperm, a critical step that directly impacts the efficacy of the procedure. This proof-of-concept study evaluated the effect of HyperSperm, a novel sperm preparation technique designed to enhance sperm function, on fertilization, embryo development, and pregnancy outcomes in both a mouse model and a first-in-human trial following IVF. In mice, HyperSperm treatment significantly increased hyperactivated motility (p < 0.05), leading to improved fertilization and blastocyst development (p < 0.05), as well as higher implantation rates (p < 0.05) and larger litter sizes (p < 0.05). Offspring displayed normal growth and fertility. In human sperm samples from normozoospermic men, HyperSperm exhibited a high safety profile, with motility, acrosome reaction, viability, and DNA fragmentation comparable to controls. A first-in-human, prospective, single-center, split-oocyte study in 10 couples undergoing IVF with donated oocytes demonstrated similar fertilization rates between HyperSperm and control groups (p = 0.425), but significantly higher usable blastocyst rates in the HyperSperm arm (43.8% vs. 67.9%, p = 0.0122). Morphokinetic parameters were comparable between groups. These results suggest that HyperSperm enhances sperm hyperactivation, a hallmark of capacitation, leading to improved embryo development in both mice and humans. This technique represents a promising approach to optimizing sperm preparation in assisted reproduction, warranting further clinical investigation.
Keywords: assisted reproductive technology; capacitation; implantation; in vitro fertilization; live birth.
Copyright © 2025 Gómez-Elías, Luque, Oscoz-Susino, Novero, Briski, Kásparas, Steeman, Stival, Lavolpe, Julianelli, Geller, Attie, Vassena, Krapf and Buffone.
Conflict of interest statement
Authors MG-E, GL, NO-S, OB, IK, RV, DK and MB were employed by Fecundis Lab. MB, DK, MG-E, GL and RV are shareholders of Fecundis. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author DK declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
-
- Alvau A., Battistone M. A., Gervasi M. G., Navarrete F. A., Xu X., Sánchez-Cárdenas C., et al. (2016). The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm. Dev. (Cambrigde) 143, 2325–2333. 10.1242/dev.136499 - DOI - PMC - PubMed
-
- Banker M., Dyer S., Chambers G. M., Ishihara O., Kupka M., de Mouzon J., et al. (2021). International committee for monitoring assisted reproductive technologies (ICMART): world report on assisted reproductive technologies, 2013. Fertil. Steril. 116, 741–756. 10.1016/J.FERTNSTERT.2021.03.039 - DOI - PubMed
LinkOut - more resources
Full Text Sources

