Characterization of cancer-associated fibroblasts and their spatial architecture reveals heterogeneity and survival associations in classic Hodgkin lymphoma
- PMID: 40433553
- PMCID: PMC12107116
- DOI: 10.1002/hem3.70145
Characterization of cancer-associated fibroblasts and their spatial architecture reveals heterogeneity and survival associations in classic Hodgkin lymphoma
Abstract
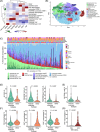
Cancer-associated fibroblasts (CAFs) are a heterogeneous population of stromal cells, which modulate the immune system and can have both pro- and anti-tumorigenic effects. In classic Hodgkin lymphoma (cHL), the role of CAFs has remained largely undefined. We applied multiplexed immunofluorescence imaging and spatial analysis on tumor samples from two independent cHL patient cohorts (n = 131 and n = 148) to study CAFs and their interactions with Hodgkin Reed-Sternberg (HRS) and tumor microenvironment (TME) cells at the single-cell resolution. We show that higher proportions of CAFs are associated with favorable outcomes, independent of the clinical covariables. In contrast, a subset of CD45+ immune cells with strong fibroblast-activation protein positivity, classified as macrophages, was less abundant in nodular sclerosis subtype and associated with worse outcomes. Neighborhood analysis allowed for the identification of colocalization or regional exclusion of phenotypically defined cell types and recurrent cellular neighborhoods. Despite the positive impact of CAF proportions on survival, patients with enrichment of platelet-derived growth factor receptor beta (PDGFRB)-positive CAFs in the vicinity of HRS cells had worse survival in both cohorts, independent of the clinical determinants. Our findings distinguish various subsets of CAFs and macrophages impacting survival in cHL and underscore the importance of the spatial arrangements in the TME.
© 2025 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
S. L. declares the following competing financial interests: consultancy fees from AbbVie, Genmab, Gilead, Incyte, Novartis, Roche, and SOBI, all outside the submitted work; honoraria from Gilead, Incyte, Novartis, and SOBI; and research grants from Bayer, Celgene/BMS, Hutchmed, Genmab, Novartis, and Roche, all outside the submitted work. C. S. declares consultancy for Bayer and Eisai and research support from Epizyme and Trillium Therapeutics Inc, all outside the submitted work.
Figures





References
-
- Aoki T, Chong LC, Takata K, et al. Single‐cell transcriptome analysis reveals disease‐defining T‐cell subsets in the tumor microenvironment of classic hodgkin lymphoma. Cancer Discov. 2020;10(3):406‐421. 10.1158/2159-8290.CD-19-0680 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
