Olive Oil Solution of Volatile Oil from Citri Reticulatae Pericarpium Viride Alleviates Slow-Transit Constipation via Regulating SCF/c-Kit Signaling Pathway and Intestinal Flora
- PMID: 40433571
- PMCID: PMC12107393
- DOI: 10.2147/DDDT.S517114
Olive Oil Solution of Volatile Oil from Citri Reticulatae Pericarpium Viride Alleviates Slow-Transit Constipation via Regulating SCF/c-Kit Signaling Pathway and Intestinal Flora
Abstract
Objective: The aroma of the aromatic class of traditional Chinese medicines can promote gastrointestinal peristalsis. This study aimed to explore the mechanisms by which volatile oil from Citri Reticulatae Pericarpium Viride (VOCRPV) alleviates slow-transit constipation (STC).
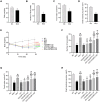
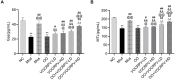
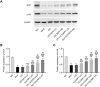
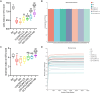
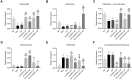
Methods: The main active ingredients in VOCRPV were determined by High-Performance Liquid Chromatography (HPLC). Due to poor stability, an olive oil solution was prepared to enhance the volatile oil's stability. A mouse model of STC was induced using loperamide hydrochloride. The mice's body weight was monitored weekly. The number of fecal pellets, fecal water content, and small intestinal propulsion rate were detected. The colon tissues were analyzed using HE staining. The serum content of gastrointestinal hormones was measured using the corresponding ELISA kit. The protein expressions of stem cell factor (SCF) and c-Kit in colon tissues were detected by Western blot and immunohistochemistry methods. The 16S rRNA gene sequencing was used to detect the intestinal flora.
Results: The contents of p-isopropyl toluene, γ-Terpinene, and d-Limonene were determined by HPLC. VOCRPV and its olive oil solution significantly enhanced body weight, increased the number of fecal pellets, improved fecal water content, and boosted small intestinal propulsion rate in mice with loperamide-induced STC, while also repairing colon mucosa damage. They also increased gastrin (Gas) and motilin (MTL) levels in treated mice, upregulated the expression of SCF and c-Kit proteins, and restored intestinal flora balance in STC mice.
Conclusion: VOCRPV could effectively alleviate STC, and olive oil enhances its therapeutic effect. VOCRPV alleviates STC by elevating Gas and MTL levels, activating the SCF/c-Kit signaling pathway, and modulating intestinal flora.
Keywords: intestinal flora; olive oil; slow-transit constipation; stem cell factor/c-Kit signaling pathway; volatile oil from Citri Reticulatae Pericarpium Viride.
© 2025 Zou et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures







Similar articles
-
Transdermal Administration of Volatile Oil from Citrus aurantium-Rhizoma Atractylodis Macrocephalae Alleviates Constipation in Rats by Altering Host Metabolome and Intestinal Microbiota Composition.Oxid Med Cell Longev. 2022 Jan 18;2022:9965334. doi: 10.1155/2022/9965334. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35087623 Free PMC article.
-
Bifidobacterium animalis subsp. lactis BLa80 alleviates constipation in mice through modulating the stem cell factor (SCF)/c-Kit pathway and the gut microbiota.Food Funct. 2025 Mar 17;16(6):2347-2362. doi: 10.1039/d4fo06350c. Food Funct. 2025. PMID: 39992179
-
[Network pharmacology research on high frequency use of Pericarpium Citri Reticulatae and Aucklandiae Radix herb pair in treatment of constipation with aromatic traditional Chinese medicine based on data mining].Zhongguo Zhong Yao Za Zhi. 2020 May;45(9):2103-2114. doi: 10.19540/j.cnki.cjcmm.20200221.304. Zhongguo Zhong Yao Za Zhi. 2020. PMID: 32495559 Chinese.
-
Prunus persica (L.) Batsch blossom soluble dietary fiber synergia polyphenol improving loperamide-induced constipation in mice via regulating stem cell factor/C-kit, NF-κB signaling pathway and gut microbiota.Food Res Int. 2024 Sep;192:114761. doi: 10.1016/j.foodres.2024.114761. Epub 2024 Jul 14. Food Res Int. 2024. PMID: 39147543
-
[Prediction of Q-markers of Citri Reticulatae Pericarpium volatile oil and GC-MS based quantitative analysis].Zhongguo Zhong Yao Za Zhi. 2021 Dec;46(24):6403-6409. doi: 10.19540/j.cnki.cjcmm.20210924.302. Zhongguo Zhong Yao Za Zhi. 2021. PMID: 34994132 Chinese.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

