Effect of Topical Timolol on Healing of Immature Breast Scars After Mammoplasty: A Randomized Controlled Trial With Blinded Assessors and Patients
- PMID: 40433711
- PMCID: PMC12118336
- DOI: 10.1111/jocd.70261
Effect of Topical Timolol on Healing of Immature Breast Scars After Mammoplasty: A Randomized Controlled Trial With Blinded Assessors and Patients
Abstract
Introduction: Wound healing is a complex process encompassing four main stages: hemostasis, inflammation, cell proliferation, maturation, and differentiation. Timolol (TM) may influence these stages, particularly re-epithelialization. This study aims to evaluate the 1-month effects of timolol on acute surgical wounds in post-mammoplasty patients.
Objectives: To investigate the efficacy of topical timolol in improving postoperative breast scars, aiming to guide future treatment protocols and prescriptions.
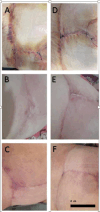
Methods: A total of 12 patients who underwent bilateral mammoplasty were enrolled in this double-blind randomized clinical trial. Treatment commenced 48 h post-surgery; one breast was treated with 0.5% timolol eye drops, while the contralateral breast received distilled water (control). Patients were advised to minimize sun exposure and pressure on the treated area, and no additional oral or topical medications were prescribed. Cleansing with a prescribed cleanser occurred every 3 days. Cosmetic assessments were conducted by a specialist at 10 and 30 days post-surgery using a 10-point Likert scale. Data were analyzed using two-way repeated measures ANOVA.
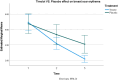
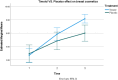
Results: Timolol significantly reduced erythema over time (Interaction, p < 0.0001; Treatment, p = 0.02), with an average decrease of 5.38 points (95% CI: 4.22-6.55) compared to 4.41 points (95% CI: 3.83-5) for placebo. The difference in reduction was 0.972 points (95% CI: 0.18-1.7). A significant improvement in the aesthetic appearance of the breast was also noted (Interaction, p < 0.0001; Treatment, p = 0.015), with timolol enhancing the aesthetic score by approximately 5.5 points (95% CI: 4.9-6.2) versus 4.58 points (95% CI: 3.4-5.7) for the placebo. Overall, timolol improved the aesthetic score by 0.972 points (95% CI: 0.23-1.7) more than the placebo.
Conclusion: Topical application of 0.5% timolol significantly improved the aesthetic appearance and reduced erythema of post-mammoplasty breast scars over a 1-month period. The results demonstrate a measurable clinical benefit, with statistically significant differences favoring timolol over placebo. These findings suggest that early intervention with topical timolol may offer a safe, effective, and non-invasive option for optimizing scar outcomes in surgical patients.
Keywords: RCT; clinical trial; immature scar; mammoplasty; postoperative scar; scar; timolol; topical timolol; wound.
© 2025 The Author(s). Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
Transparency declaration: Authors declare that the manuscript is honest, accurate, and transparent. No important aspect of the study is omitted.
The authors declare no conflicts of interest.
Figures


Similar articles
-
Efficacy and safety of topical timolol 0.5% plus saline 0.9% versus each one alone in acne scar trichloroacetic acid-CROSS therapy: A blinded randomized controlled trial.Dermatol Ther. 2022 Apr;35(4):e15341. doi: 10.1111/dth.15341. Epub 2022 Feb 27. Dermatol Ther. 2022. PMID: 35094447 Clinical Trial.
-
Topical calcipotriol for preventive treatment of hypertrophic scars: a randomized, double-blind, placebo-controlled trial.Arch Dermatol. 2009 Nov;145(11):1269-75. doi: 10.1001/archdermatol.2009.237. Arch Dermatol. 2009. PMID: 19917956 Clinical Trial.
-
Short-term Efficacy and Safety of Topical β-Blockers (Timolol Maleate Ophthalmic Solution, 0.5%) in Acute Migraine: A Randomized Crossover Trial.JAMA Ophthalmol. 2020 Nov 1;138(11):1160-1166. doi: 10.1001/jamaophthalmol.2020.3676. JAMA Ophthalmol. 2020. PMID: 33001159 Free PMC article. Clinical Trial.
-
A systematic review of comparative clinical trials on the efficacy, safety, and patient satisfaction of ablative and non-ablative laser therapies for atrophic, hypertrophic, and keloid scars.Lasers Med Sci. 2025 Jun 14;40(1):280. doi: 10.1007/s10103-025-04519-3. Lasers Med Sci. 2025. PMID: 40515775 Review.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
References
-
- Roohaninasab M., Khodadad F., Sadeghzadeh‐Bazargan A., et al., “Efficacy of Fractional CO2 Laser in Combination With Stromal Vascular Fraction (SVF) Compared With Fractional CO2 Laser Alone in the Treatment of Burn Scars: A Randomized Controlled Clinical Trial,” Stem Cell Research & Therapy 14, no. 1 (2023): 269, 10.1186/s13287-023-03480-8. - DOI - PMC - PubMed
-
- Roohaninasab M., Jafarzadeh A., Sadeghzadeh‐Bazargan A., et al., “Evaluation of the Efficacy, Safety and Satisfaction Rates of Platelet‐Rich Plasma, Non‐Cross‐Linked Hyaluronic Acid and the Combination of Platelet‐Rich Plasma and Non‐Cross‐Linked Hyaluronic Acid in Patients With Burn Scars Treated With Fractional CO2 Laser: A Randomized Controlled Clinical Trial,” International Wound Journal 21, no. 10 (2024): e70065, 10.1111/iwj.70065. - DOI - PMC - PubMed
-
- Potaliya P., Nayak P., and Goyal M., “In‐Vitro Wound Healing Assays: A Comprehensive Review,” Mymensingh Medical Journal 33, no. 2 (2024): 613–625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

